Heterogeneous Electrofreezing Triggered by CO2 on Pyroelectric Crystals: Qualitatively Different Icing on Hydrophilic and Hydrophobic Surfaces
Dedicated to the memory of Professor Duilio Arigoni (1928–2020)
Graphical Abstract
Abstract
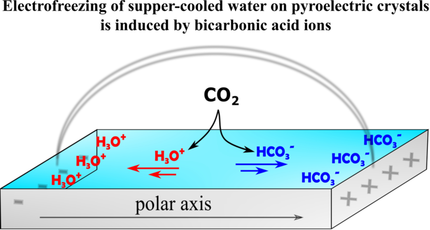
By performing icing experiments on hydrophilic and hydrophobic surfaces of pyroelectric amino acids and on the x-cut faces of LiTaO3, we discovered that the effect of electrofreezing of super cooled water is triggered by ions of carbonic acid. During the cooling of the hydrophilic pyroelectric crystals, a continuous water layer is created between the charged hemihedral faces, as confirmed by impedance measurements. As a result, a current of carbonic acid ions, produced by dissolved environmental CO2, flows through the wetted layer towards the hemihedral faces and elevates the icing temperature. This proposed mechanism is based on the following: (i) on hydrophilic surfaces, water with dissolved CO2 (pH 4) freezes at higher temperatures than pure water of pH 7. (ii) In the absence of the ionic current, achieved by linking the two hemihedral faces of hydrophilic crystals by a conductive paint, water of the two pH levels freeze at the same temperature. (iii) On hydrophobic crystals with similar pyroelectric coefficients, where there is no continuous wetted layer, no electrofreezing effect is observed.