Hydrogenolysis of Polysilanes Catalyzed by Low-Valent Nickel Complexes
Bruno Pribanic
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich, Switzerland
Search for more papers by this authorCorresponding Author
Monica Trincado
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich, Switzerland
Search for more papers by this authorFrederik Eiler
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich, Switzerland
Search for more papers by this authorMatthias Vogt
Universität Bremen, Fachbereich 2 Biologie/Chemie, Institut für Anorganische Chemie und Kristallographie, Leobenerstr. 7, 28359 Bremen, Germany
Search for more papers by this authorAleix Comas-Vives
Chemistry Department, Universitat Autònoma de Barcelona, Cerdanyola del Vallès, 08193 Bellaterra, Catalonia, Spain
Search for more papers by this authorCorresponding Author
Hansjörg Grützmacher
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich, Switzerland
Search for more papers by this authorBruno Pribanic
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich, Switzerland
Search for more papers by this authorCorresponding Author
Monica Trincado
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich, Switzerland
Search for more papers by this authorFrederik Eiler
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich, Switzerland
Search for more papers by this authorMatthias Vogt
Universität Bremen, Fachbereich 2 Biologie/Chemie, Institut für Anorganische Chemie und Kristallographie, Leobenerstr. 7, 28359 Bremen, Germany
Search for more papers by this authorAleix Comas-Vives
Chemistry Department, Universitat Autònoma de Barcelona, Cerdanyola del Vallès, 08193 Bellaterra, Catalonia, Spain
Search for more papers by this authorCorresponding Author
Hansjörg Grützmacher
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich, Switzerland
Search for more papers by this authorDedicated to Professor José Manuel González Díaz on the occasion of his 60th birthday
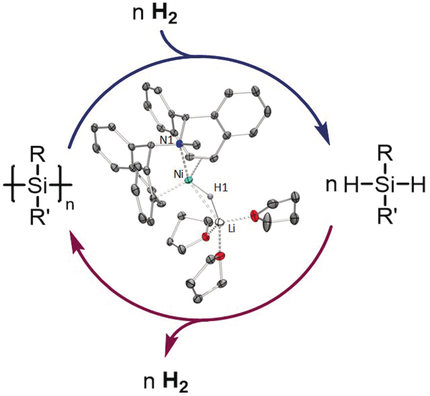
Graphical Abstract
Si−Si bonds in polysilanes can be converted to Si−H bonds under mild conditions by using low-valent anionic Ni0 hydride catalysts. The reaction is reversible and dehydrogenative coupling of hydrosilanes is also possible. Experiments and DFT calculations indicate that low-coordinated 14- and 16-electron d10-Ni hydrides, silyl, and silane intermediates are involved in the catalytic cycle.
Abstract
The dehydrogenation of organosilanes (RxSiH4−x) under the formation of Si−Si bonds is an intensively investigated process leading to oligo- or polysilanes. The reverse reaction is little studied. To date, the hydrogenolysis of Si−Si bonds requires very harsh conditions and is very unselective, leading to multiple side products. Herein, we describe a new catalytic hydrogenation of oligo- and polysilanes that is highly selective and proceeds under mild conditions. New low-valent nickel hydride complexes are used as catalysts and secondary silanes, RR′SiH2, are obtained as products in high purity.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201907525-sup-0001-misc_information.pdf7.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1I. Ojima in The Chemistry of Organic Silicon Compounds (Eds.: ), Wiley Interscience, New York, 1989, pp. 1479–1526.
10.1002/0470025107.ch25 Google Scholar
- 2A. M. Tondreau, C. C. Hojilla Atienza, K. J. Weller, S. A. Nye, K. M. Lewis, J. G. P. Delis, P. J. Chirik, Science 2012, 335, 567–570.
- 3K. H. Chung, N. Yao, J. Benziger, J. C. Sturm, K. K. Singh, D. Carlson, S. Kuppurao, Appl. Phys. Lett. 2008, 92, 113506.
- 4H. Habuka, T. Masaki, Surf. Coat. Technol. 2013, 217, 88–93.
- 5M. Oestreich, A. Simonneau, WO 2015036309, A1 20150319, 2015.
- 6R. Müller, VEB Silikonchemie, DD 5448, 1942.
- 7E. G. Rochow, USP 2380995, 1941.
- 8A. J. Barry, USP 2499561, 1950.
- 9B. Marciniec, Hydrosilylation: A comprehensive review on recent advances, Springer, Amsterdam, 2009.
10.1007/978-1-4020-8172-9 Google Scholar
- 10B. Arkles, in Handbook of Grignard Reagents, Grignard reagents and silanes (Eds.: ), Marcel Dekker Inc., New York, 1966, pp. 667–675.
- 11Wacker Chemie AG, US5502230A, 1996.
- 12
- 12aUnion Carbide Corporation, US2606811A, 1952;
- 12bRhodia Chimie, FR99/00771, 1999;
- 12cDow Corning Corporation, US6013235A, 2000;
- 12dWacker Chemie AG, DE102007055732A1, 2007.
- 13W. J. Schulz, USP 5015624, 1991.
- 14R. D. Miller, J. Michl, Chem. Rev. 1989, 89, 1359–1410.
- 15A. Feigl, A. Bockholt, J. Weis, B. Rieger in Modern synthetic and application aspects of polysilanes: An underestimated class of materials? Silicon Polymers. (Ed.: ), Springer, Berlin, Heidelberg, 2011, pp. 1–31.
- 16J. Y. Corey, Adv. Organomet. Chem. 2004, 51, 1–52.
- 17M. C. Lipke, A. L. Liberman-Martin, T. D. Tilley, Angew. Chem. Int. Ed. 2017, 56, 2260–2294; Angew. Chem. 2017, 129, 2298–2335.
- 18
- 18aG. I. Nikonov, Adv. Organomet. Chem. 2005, 53, 217;
- 18bS. Lachaize, S. Sabo-Etienne, Eur. J. Inorg. Chem. 2006, 2115–2127;
- 18cJ. Y. Corey, Chem. Rev. 2011, 111, 863–1071;
- 18dJ. Y. Corey, Chem. Rev. 2016, 116, 11291–11435.
- 19F. G. Fontaine, T. Kadkhodazadeh, D. Zargarian, Chem. Commun. 1998, 1253–1254.
- 20E. E. Smith, G. D. Du, P. E. Fanwick, M. M. Abu-Omar, Organometallics 2010, 29, 6527–6533.
- 21M. Tanabe, A. Takahashi, T. Fukuta, K. Osakada, Organometallics 2013, 32, 1037–1043.
- 22Y. R. Luo in Handbook of bond dissociation energies in organic compounds, CRC Press, Boca Raton, 2003, p. 380.
- 23L. Rosenberg, C. W. Davis, J. Yao, J. Am. Chem. Soc. 2001, 123, 5120–5121.
- 24T. H. Dunning, J. Chem. Phys. 1989, 90, 1007.
- 25J. Voigt, M. A. Chilleck, T. Braun, Dalton Trans. 2013, 42, 4052–4058.
- 26S. I. Kalläne, R. Laubenstein, T. Braun, M. Dietrich, Eur. J. Inorg. Chem. 2016, 530–537.
- 27D. Schmidt, T. Zell, T. Schaub, U. Radius, Dalton Trans. 2014, 43, 10816–10827.
- 28For a review on nickel hydride chemistry, see: N. A. Eberhardt, H. Guan, Chem. Rev. 2016, 116, 8373–8426.
- 29P. Kelley, S. Lin, G. Edouard, M. W. Day, T. Agapie, J. Am. Chem. Soc. 2012, 134, 5480–5483.
- 30M. L. Helm, M. P. Stewart, R. M. Bullock, M. R. DuBois, D. A. DuBois, Science 2011, 333, 863–866.
- 31
- 31aM. O'Hagan, W. J. Shaw, S. Raugei, S. Chen, J. Y. Yang, U. J. Kilgore, L. DuBois, R. M. Bullock, J. Am. Chem. Soc. 2011, 133, 14301–14312;
- 31bD. Schilter, J. M. Camara, M. T. Huynh, S. Hammes-Schiffer, T. B. Rauchfuss, Chem. Rev. 2016, 116, 8693–8749;
- 31cC. Finazzo, J. Harmer, C. Bauer, B. Jaun, E. C. Duin, F. Mahlert, M. Goenrich, R. K. Thauer, S. Van Doorslaer, A. Schweiger, J. Am. Chem. Soc. 2008, 130, 10907–10920.
- 32
- 32aK. R. Pörschke, W. G. Kleirnann, K. H. Claus, C. Krüger, Angew. Chem. Int. Ed. Engl. 1983, 22, 991–992; Angew. Chem. 1983, 95, 1032–1033;
- 32bK. R. Pörschke, Thermolabile Hydridio- und Organonickel(0)-Verbindungen. Habilitationsschrift, Universität Düsseldorf, 1988.
- 33G. Longoni, M. Manassero, M. Sansoni, J. Organomet. Chem. 1979, 174, C41–C44.
- 34R. C. Cammarota, M. V. Vollmer, J. Xie, J. Ye, J. C. Linehan, S. A. Burgess, A. M. Appel, L. Gagliardi, C. Lu, J. Am. Chem. Soc. 2017, 139, 14244–14250.
- 35M. Vogt, B. de Bruin, H. Berke, M. Trincado, H. Grützmacher, Chem. Sci. 2011, 2, 723–727.
- 36X. Yang, T. L. Gianetti, J. Harbort, M. D. Wörle, L. Tan, C.-Y. Su, P. Jurt, J. R. Harmer, H. Grützmacher, Angew. Chem. Int. Ed. 2016, 55, 11999–12002; Angew. Chem. 2016, 128, 12178–12181.
- 37T. Büttner, J. Geier, G. Frison, J. Harmer, C. Calle, A. Schweiger, H. Schönberg, H. Grützmacher, Science 2005, 307, 235–238.
- 38The proton spin-lattice relaxation times (T1) found for the Ni−H resonances in hydride complexes 5 b (508(±4.2) ms) and 6 b (662(±6.3) ms) indicate classical hydride species.
- 39“Noncovalent metal–metal interactions”: K. M. C. Wong, V. K. M. Au, V. W. W. Yam, in Comprehensive Inorganic Chemistry II, 2nd ed. ), Elsevier, Amsterdam, 2013, pp. 59–130.
- 40See, for example: V. M. Iluc, G. L. Hillhouse, J. Am. Chem. Soc. 2010, 132, 11890–11892.
- 41S. Wu, X. Li, Z. Xiong, W. Xu, Y. Lu, H. Sun, Organometallics 2013, 32, 3227–3237.
- 42J. Takaya, N. Iwasawa, Dalton Trans. 2011, 40, 8814–8821.
- 43
- 43aR. F. W. Bader, C. F. Matta, F. Cortés-Guzmán, Organometallics 2004, 23, 6253–6263;
- 43bC. F. Matta, R. J. Boyd in The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design (Eds.: ), Wiley, Hoboken, 2007, Chapter 1. An Introduction to the Quantum Theory of Atoms in Molecules, pp. 1–34.
10.1002/9783527610709.ch1 Google Scholar
- 44D. Cremer, E. Kraka, Angew. Chem. Int. Ed. Engl. 1984, 23, 627–628; Angew. Chem. 1984, 96, 612–614.
- 45R. Bianchi, G. Gervasio, D. Marabello, Inorg. Chem. 2000, 39, 2360–2366.
- 46F. Uhlig, P. Gspaltl, M. Trabi, E. Hengge, J. Organomet. Chem. 1995, 493, 33–40.