Migratory Arylboration of Unactivated Alkenes Enabled by Nickel Catalysis
Wang Wang
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorChao Ding
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorYangyang Li
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorZheqi Li
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorYuqiang Li
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorLong Peng
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guoyin Yin
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorWang Wang
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorChao Ding
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorYangyang Li
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorZheqi Li
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorYuqiang Li
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorLong Peng
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guoyin Yin
The Institute for Advanced Studies, Wuhan University, Wuhan, 430072 P. R. China
Search for more papers by this authorGraphical Abstract
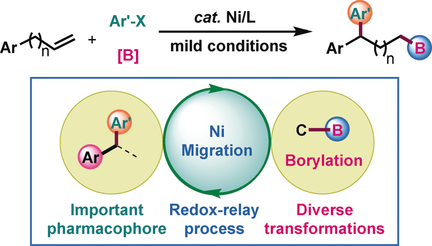
An unprecedented arylboration of unactivated terminal alkenes, featuring 1,n-regioselectivity (n>2), has been achieved by nickel catalysis. An array of valuable alkylboronic esters are prepared with this method. Mechanism studies indicate that a nickel migration and a selective bond-formation step are involved in this reaction.
Abstract
An unprecedented arylboration of unactivated terminal alkenes, featuring 1,n-regioselectivity, has been achieved by nickel catalysis. The nitrogen-based ligand plays an essential role in the success of this three-component reaction. This transformation displays good regioselectivity and excellent functional-group tolerance. In addition, the incorporation of a boron group into the products provides substantial opportunities for further transformations. Also demonstrated is that the products can be readily transformed into pharmaceutically relevant molecules. Unexpectedly, preliminary mechanistic studies indicate that although the metal migration favors the α-position of boron, selective and decisive bond formation is favored at the benzylic position.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201814572-sup-0001-misc_information.pdf11.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews on alkene difunctionalization:
- 1aR. I. McDonald, G. Liu, S. S. Stahl, Chem. Rev. 2011, 111, 2981–3019;
- 1bV. Saini, B. J. Stokes, M. S. Sigman, Angew. Chem. Int. Ed. 2013, 52, 11206–11220; Angew. Chem. 2013, 125, 11414–11429;
- 1cG. Yin, X. Mu, G. Liu, Acc. Chem. Res. 2016, 49, 2413–2423;
- 1dJ. R. Coombs, J. P. Morken, Angew. Chem. Int. Ed. 2016, 55, 2636–2649; Angew. Chem. 2016, 128, 2682–2696;
- 1eJ. Derosa, V. T. Tran, V. A. Van der Puyl, K. M. Engle, Chem. Sci. 2018, 9, 5278–5283.
- 2For reviews, see:
- 2aK. Semba, T. Fujihara, J. Terao, Y. Tsuji, Tetrahedron 2015, 71, 2183–2197;
- 2bE. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott, T. B. Marder, Chem. Rev. 2016, 116, 9091–9161;
- 2cD. Hemming, R. Fritzemeier, S. A. Westcott, W. L. Santos, P. G. Steel, Chem. Soc. Rev. 2018, 47, 7477–7494.
- 31,2-arylboration by dual metal catalysis:
- 3aK. Semba, Y. Nakao, J. Am. Chem. Soc. 2014, 136, 7567–7570;
- 3bK. B. Smith, K. M. Logan, W. You, M. K. Brown, Chem. Eur. J. 2014, 20, 12032–12036;
- 3cK. M. Logan, K. B. Smith, M. K. Brown, Angew. Chem. Int. Ed. 2015, 54, 5228–5231; Angew. Chem. 2015, 127, 5317–5320;
- 3dK. Semba, Y. Ohtagaki, Y. Nakao, Org. Lett. 2016, 18, 3956–3959;
- 3eK. M. Logan, M. K. Brown, Angew. Chem. Int. Ed. 2017, 56, 851–855; Angew. Chem. 2017, 129, 869–873;
- 3fK. B. Smith, M. K. Brown, J. Am. Chem. Soc. 2017, 139, 7721–7724;
- 3gS. R. Sardini, M. K. Brown, J. Am. Chem. Soc. 2017, 139, 9823–9826;
- 3hB. Chen, P. Cao, X. Yin, Y. Liao, L. Jiang, J. Ye, M. Wang, J. Liao, ACS Catal. 2017, 7, 2425–2429.
- 4For a review, see: D. R. Pye, N. P. Mankad, Chem. Sci. 2017, 8, 1705–1718.
- 5Examples on alkene 1,2-arylborations with palladium catalysis:
- 5aK. Yang, Q. Song, J. Org. Chem. 2016, 81, 1000–1005;
- 5bK. Yang, Q. Song, Org. Lett. 2016, 18, 5460–5463.
- 6A single example on alkene 1,2-arylborations with nickel catalysis: K. M. Logan, S. R. Sardini, S. D. White, M. K. Brown, J. Am. Chem. Soc. 2018, 140, 159–162.
- 7For reviews, see:
- 7aA. Vasseur, J. Bruffaerts, I. Marek, Nat. Chem. 2016, 8, 209–219;
- 7bH. Sommer, F. Juliá-Hernández, R. Martin, I. Marek, ACS Cent. Sci. 2018, 4, 153–165; examples on 1,3-difunctionalization of alkenes involving metal migration:
- 7cR. T. Thornbury, V. Saini, T. A. Fernandes, C. B. Santiago, E. P. A. Talbot, M. S. Sigman, J. M. McKenna, F. D. Toste, Chem. Sci. 2017, 8, 2890–2897;
- 7dW. Li, J. K. Boon, Y. Zhao, Chem. Sci. 2018, 9, 600–607;
- 7eP. Basnet, R. K. Dhungana, S. Thapa, B. Shrestha, K. C. Shekhar, J. M. Sears, R. Giri, J. Am. Chem. Soc. 2018, 140, 7782–7786.
- 8Examples on alkene 1,1-arylboration reactions:
- 8aH. M. Nelson, B. D. Williams, J. Miró, F. D. Toste, J. Am. Chem. Soc. 2015, 137, 3213–3216;
- 8bA. M. Bergmann, S. K. Dorn, K. B. Smith, K. M. Logan, M. K. Brown, Angew. Chem. Int. Ed. 2019, 58, 1719–1723; Angew. Chem. 2019, 131, 1733–1737.
- 9Another strategy for 1,n-difunctionalization of alkenes is by 1,5-H shift. For examples, see:
- 9aP. Yu, J.-S. Lin, L. Li, S.-C. Zheng, Y.-P. Xiong, L.-J. Zhao, B. Tan, X.-Y. Liu, Angew. Chem. Int. Ed. 2014, 53, 11890–11894; Angew. Chem. 2014, 126, 12084–12088;
- 9bP. Yu, S.-C. Zheng, N.-Y. Yang, B. Tan, X.-Y. Liu, Angew. Chem. Int. Ed. 2015, 54, 4041–4045; Angew. Chem. 2015, 127, 4113–4117.
- 10
- 10aS. Li, K. Huang, J. Zhang, W. Wu, X. Zhang, Org. Lett. 2013, 15, 1036–1039;
- 10bY. Yin, Y. Dai, H. Jia, J. Li, L. Bu, B. Qiao, X. Zhao, Z. Jiang, J. Am. Chem. Soc. 2018, 140, 6083–6087.
- 11
- 11aL. Peng, Y. Li, Y. Li, W. Wang, H. Pang, G. Yin, ACS Catal. 2018, 8, 310–313;
- 11bL. Peng, Z. Li, Org. Lett. 2018, 20, 1880–1883.
- 12Leading examples on nickel chain-walking:
- 12aJ. S. Bair, Y. Schramm, A. G. Sergeev, E. Clot, O. Eisenstein, J. F. Hartwig, J. Am. Chem. Soc. 2014, 136, 13098–13101;
- 12bI. Buslov, J. Becouse, S. Mazza, M. Montandon-Clerc, X. Hu, Angew. Chem. Int. Ed. 2015, 54, 14523–14526; Angew. Chem. 2015, 127, 14731–14734;
- 12cF. Juliá-Hernández, T. Moragas, J. Cornella, R. Martin, Nature 2017, 545, 84–88;
- 12dY. He, Y. Cai, S. Zhu, J. Am. Chem. Soc. 2017, 139, 1061–1064;
- 12eF. Zhou, J. Zhu, Y. Zhang, S. Zhu, Angew. Chem. Int. Ed. 2018, 57, 4058–4062; Angew. Chem. 2018, 130, 4122–4126.
- 13F.-S. Han, Chem. Soc. Rev. 2013, 42, 5270–5298.
- 14W. B. Reid, J. J. Spillane, S. B. Krause, D. A. Watson, J. Am. Chem. Soc. 2016, 138, 5539–5542.
- 15L. Li, T. Gong, B. Xiao, Y. Fu, Nat. Commun. 2017, 8, 345.
- 16B.-L. Lin, L. Liu, Y. Fu, S.-W. Luo, Q. Chen, Q.-X. Guo, Organometallics 2004, 23, 2114–2123.
- 17Examples on branched selectivity in nickel catalysis:
- 17aR. Matsubara, A. C. Gutierrez, T. F. Jamison, J. Am. Chem. Soc. 2011, 133, 19020–19023;
- 17bS. Z. Tasker, A. C. Gutierrez, T. F. Jamison, Angew. Chem. Int. Ed. 2014, 53, 1858–1861; Angew. Chem. 2014, 126, 1889–1892.
- 18A review on the PyrOx ligand: G. Yang, W. Zhang, Chem. Soc. Rev. 2018, 47, 1783–1810.
- 19When a chiral L5 was used, <5 % ee was observed.
- 20Benzyl-NiII species probably have a similar reactivity to that of allyl-NiII species. For leading references on allyl-NiII species reacting with eletrophiles, see:
- 20aL. S. Hegedus, D. H. P. Thompson, J. Am. Chem. Soc. 1985, 107, 5663–5668;
- 20bR. Shrestha, S. C. M. Dorn, D. J. Weix, J. Am. Chem. Soc. 2013, 135, 751–762.