Copper-Mediated Aminoquinoline-Directed Radiofluorination of Aromatic C−H Bonds with K18F
Dr. So Jeong Lee
Department of Radiology, University of Michigan, 1301 Catherine St, Ann Arbor, MI, 48109 USA
Search for more papers by this authorKatarina J. Makaravage
Department of Chemistry, The University of Michigan, 930 North University Ave, Ann Arbor, MI, 48109 USA
Search for more papers by this authorDr. Allen F. Brooks
Department of Radiology, University of Michigan, 1301 Catherine St, Ann Arbor, MI, 48109 USA
Search for more papers by this authorCorresponding Author
Prof. Peter J. H. Scott
Department of Radiology, University of Michigan, 1301 Catherine St, Ann Arbor, MI, 48109 USA
Search for more papers by this authorCorresponding Author
Prof. Melanie S. Sanford
Department of Chemistry, The University of Michigan, 930 North University Ave, Ann Arbor, MI, 48109 USA
Search for more papers by this authorDr. So Jeong Lee
Department of Radiology, University of Michigan, 1301 Catherine St, Ann Arbor, MI, 48109 USA
Search for more papers by this authorKatarina J. Makaravage
Department of Chemistry, The University of Michigan, 930 North University Ave, Ann Arbor, MI, 48109 USA
Search for more papers by this authorDr. Allen F. Brooks
Department of Radiology, University of Michigan, 1301 Catherine St, Ann Arbor, MI, 48109 USA
Search for more papers by this authorCorresponding Author
Prof. Peter J. H. Scott
Department of Radiology, University of Michigan, 1301 Catherine St, Ann Arbor, MI, 48109 USA
Search for more papers by this authorCorresponding Author
Prof. Melanie S. Sanford
Department of Chemistry, The University of Michigan, 930 North University Ave, Ann Arbor, MI, 48109 USA
Search for more papers by this authorGraphical Abstract
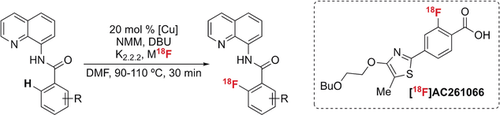
Late-stage fluorination: K18F is applied in a newly developed Cu-mediated ortho-C(sp2)−H radiofluorination of aromatic carboxylic acids that are protected as 8-aminoquinoline benzamides. Fluorination of 18 examples in up to 62 % radiochemical yield and high specific activity is reported, including the automated synthesis of [18F]AC261066.
Abstract
A Cu-mediated ortho-C−H radiofluorination of aromatic carboxylic acids that are protected as 8-aminoquinoline benzamides is described. The method uses K18F and is compatible with a wide range of functional groups. The reaction is showcased in the high specific activity automated synthesis of the RARβ2 agonist [18F]AC261066.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201812701-sup-0001-misc_information.pdf25.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2014, 114, 2432;
- 1bE. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly, N. A. Meanwell, J. Med. Chem. 2015, 58, 8315.
- 2N. A. Meanwell, J. Med. Chem. 2018, 61, 5822.
- 3S. M. Ametamey, M. Honer, P. A. Schubiger, Chem. Rev. 2008, 108, 1501.
- 4
- 4aM. M. Alauddin, Am. J. Nucl. Med. Mol. Imaging 2012, 2, 55;
- 4bP. Brust, J. van den Hoff, J. Steinbach, Neurosci. Bull. 2014, 30, 777.
- 5For recent reviews and perspectives on late-stage fluorination, see:
- 5aA. F. Brooks, J. J. Topczewski, N. Ichiishi, M. S. Sanford, P. J. H. Scott, Chem. Sci. 2014, 5, 4545;
- 5bM. G. Campbell, T. Ritter, Chem. Rev. 2015, 115, 612;
- 5cS. Preshlock, M. Tredwell, V. Gouverneur, Chem. Rev. 2016, 116, 719;
- 5dM. S. Sanford, P. J. H. Scott, ACS Cent. Sci. 2016, 2, 128;
- 5eM. G. Campbell, J. Mercier, C. Genicot, V. Gouverneur, J. M. Hooker, T. Ritter, Nat. Chem. 2017, 9, 1.
- 6
- 6aE. Lee, A. S. Kamlet, D. C. Powers, C. N. Neumann, G. B. Boursalian, T. Furuya, D. C. Choi, J. M. Hooker, T. Ritter, Science 2011, 334, 639;
- 6bE. Lee, J. M. Hooker, T. Ritter, J. Am. Chem. Soc. 2012, 134, 17456;
- 6cN. Ichiishi, A. F. Brooks, J. J. Topczewski, M. E. Rodnick, M. S. Sanford, P. J. H. Scott, Org. Lett. 2014, 16, 3224;
- 6dM. Tredwell, S. M. Preshlock, N. J. Taylor, S. Gruber, M. Huiban, J. Passchier, J. Mercier, C. Génicot, V. Gouverneur, Angew. Chem. Int. Ed. 2014, 53, 7751; Angew. Chem. 2014, 126, 7885;
- 6eA. V. Mossine, A. F. Brooks, K. J. Makaravage, J. M. Miller, N. Ichiishi, M. S. Sanford, P. J. H. Scott, Org. Lett. 2015, 17, 5780;
- 6fB. D. Zlatopolskiy, J. Zischler, P. Krapf, F. Zarrad, E. A. Urusova, E. Kordys, H. Endepols, B. Neumaier, Chem. Eur. J. 2015, 21, 5972;
- 6gK. J. Makaravage, A. F. Brooks, A. V. Mossine, M. S. Sanford, P. J. H. Scott, Org. Lett. 2016, 18, 5440;
- 6hC. N. Neumann, J. M. Hooker, T. Ritter, Nature 2016, 534, 369;
- 6iM. H. Beyzavi, D. Mandal, M. G. Strebl, C. N. Neumann, E. M. D′Amato, J. Chen, J. M. Hooker, T. Ritter, ACS Cent. Sci. 2017, 3, 944;
- 6jA. V. Mossine, A. F. Brooks, V. Bernard-Gauthier, J. J. Bailey, N. Ichiishi, R. Schirrmacher, M. S. Sanford, P. J. H. Scott, J. Labelled Compd. Radiopharm. 2018, 61, 228;
- 6kN. J. Taylor, E. Emer, S. Preshlock, M. Schedler, M. Tredwell, S. Verhoog, J. Mercier, C. Genicot, V. Gouverneur, J. Am. Chem. Soc. 2017, 139, 8267.
- 7
- 7aM. B. Nodwell, H. Yang, M. Čolović, Z. Yuanm, H. Merkens, R. E. Martin, F. Bénard, P. Schaffer, R. Britton, J. Am. Chem. Soc. 2017, 139, 3595;
- 7bZ. Yuan, M. Nodwell, H. Yang, N. Malik, H. Merkens, F. Bernard, R. Martin, P. Schaffer, R. Britton, Angew. Chem. Int. Ed. 2018, 57, 12733; Angew. Chem. 2018, 130, 12915.
- 8
- 8aX. Huang, W. Liu, H. Ren, R. Neelamegam, J. M. Hooker, J. T. Groves, J. Am. Chem. Soc. 2014, 136, 6842;
- 8bX. Huang, W. Liu, J. M. Hooker, J. T. Groves, Angew. Chem. Int. Ed. 2015, 54, 5241; Angew. Chem. 2015, 127, 5330;
- 8cW. Liu, X. Huang, M. S. Placzek, S. W. Krska, P. McQuade, J. M. Hooker, J. T. Groves, Chem. Sci. 2018, 9, 1168.
- 9O. Jacobson, D. O. Kiesewetter, X. Chen, Bioconjugate Chem. 2015, 26, 1.
- 10J. Bergman, O. Solin, Nucl. Med. Biol. 1997, 24, 677.
- 11M. S. McCammant, S. Thompson, A. F. Brooks, S. W. Krska, P. J. H. Scott, M. S. Sanford, Org. Lett. 2017, 19, 3939.
- 12T. Truong, K. Klimovica, O. Daugulis, J. Am. Chem. Soc. 2013, 135, 9342.
- 13P. J. H. Scott, A. F. Brooks, N. Ichiishi, M. S. Sanford, Preparation of Ag18F and its use in the Synthesis of PET Radiotracers, U.S. Patent 2016/0317682 A1, Nov 3, 2016.
- 14Other bases were also compatible (e.g. comparable RCCs could be obtained using 1,5-diazabicyclo[4.3.0]non-5-ene; see Supporting Information).
- 15When Table 1, entry 5 was set up in a glovebox and kept under an inert atmosphere the RCC dropped prohibitively (to 6±4 %), further consistent with the role of air as the oxidant.
- 16Product identities were confirmed by radio-HPLC. To further confirm that radiofluorination occurred at the expected ortho-site (rather than on the quinoline ring) we conducted control experiments and demonstrated baseline separation of regioisomeric products by HPLC (see Supporting Information).
- 17Some arenes bearing electron-withdrawing substituents give rise to minor side products. We ruled out the formation of side products derived from competing SNAr reactions (see Supporting Information), but have not been able to positively identity the side products to date.
- 18
- 18aJ. Ding, Y. Zhang, J. Li, Org. Chem. Front. 2017, 4, 1528;
- 18bH. Chen, P. Li, M. Wang, L. Wang, Eur. J. Org. Chem. 2018, 2091.
- 19Compounds 15–18 contain functional groups that could potentially direct C−H fluorination elsewhere in the molecule (e.g. 17H contains 2 amide groups). Small impurity peaks were detected in the crude radio-HPLC traces of these products; however, 1518F–1818F were the major products in each case, and they appear to be readily separable from the side products formed in the reaction.
- 20B. W. Lund, F. Piu, N. K. Gauthier, A. Eeg, E. Currier, V. Sherbukhin, M. R. Brann, U. Hacksell, R. Olsson, J. Med. Chem. 2005, 48, 7517.
- 21These unoptimized automation results demonstrate that this method can be used to prepare sufficient amounts of radiotracers for pre-clinical evaluation in rodents and non-human primates. We expect that yields can be further improved through careful optimization of the automated method. This work is currently underway, along with qualification of a synthesis and formulation of 2018F for preclinical use.
- 22Radioiodination of this scaffold has been reported. See: L. W. Deady, J. Desneves, L. M. A. Tilley, J. Labelled Compd. Radiopharm. 2000, 43, 977.