Structural Snapshots of Cluster Growth from {U6} to {U38} During the Hydrolysis of UCl4
Dr. Lucile Chatelain
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
Search for more papers by this authorRadmila Faizova
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Farzaneh Fadaei-Tirani
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
Search for more papers by this authorJacques Pécaut
Univ. Grenoble Alpes, CEA, CNRS, INAC, SYMMES, UMR 5819 Equipe Chimie Interface Biologie pour l'Environnement la Santé et la Toxicologie, 17 Rue des Martyrs, 38000 Grenoble, France
Search for more papers by this authorCorresponding Author
Prof. Marinella Mazzanti
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Lucile Chatelain
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
Search for more papers by this authorRadmila Faizova
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Farzaneh Fadaei-Tirani
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
Search for more papers by this authorJacques Pécaut
Univ. Grenoble Alpes, CEA, CNRS, INAC, SYMMES, UMR 5819 Equipe Chimie Interface Biologie pour l'Environnement la Santé et la Toxicologie, 17 Rue des Martyrs, 38000 Grenoble, France
Search for more papers by this authorCorresponding Author
Prof. Marinella Mazzanti
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
Search for more papers by this authorGraphical Abstract
Abstract
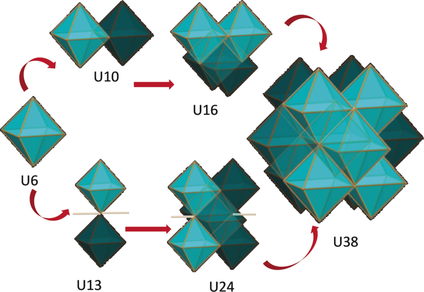
Herein we report the assembly of large uranium(IV) clusters with novel nuclearities and/or shapes from the controlled hydrolysis of UCl4 in organic solution and in the presence of the benzoate ligands. {U6}, {U13}, {U16}, {U24}, {U38} oxo and oxo/hydroxo clusters were isolated and crystallographically characterized. These structural snapshots indicate that larger clusters are slowly built from the condensation of octahedral {U6} building blocks. The uranium/benzoate ligand ratio, the reaction temperature and the presence of base play an important role in determining the structure of the final assembly. Moreover, the isolation of different size cluster {U6} (few hours), {U16} (3 days), {U24} (21 days) from the same solution in a chosen set of conditions shows that the assembly of uranium oxo clusters in hydrolytic conditions is time dependent.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201812509-sup-0001-misc_information.pdf2.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Hickam, P. C. Burns in Recent Development in Clusters of Rare Earths and Actinides: Chemistry and Materials, Vol. 173 (Ed.: ), Springer, Cham, 2017, pp. 121–153;
- 1bJ. Qiu, P. C. Burns, Chem. Rev. 2013, 113, 1097–1120;
- 1cK. E. Knope, L. Soderholm, Chem. Rev. 2013, 113, 944–994.
- 2
- 2aJ. D. Rinehart, T. D. Harris, S. A. Kozimor, B. M. Bartlett, J. R. Long, Inorg. Chem. 2009, 48, 3382–3395;
- 2bS. T. Liddle, J. van Slageren, Chem. Soc. Rev. 2015, 44, 6655–6669;
- 2cD. P. Mills, F. Moro, J. McMaster, J. van Slageren, W. Lewis, A. J. Blake, S. T. Liddle, Nat. Chem. 2011, 3, 454–460;
- 2dV. Mougel, L. Chatelain, J. Pecaut, R. Caciuffo, E. Colineau, J. C. Griveau, M. Mazzanti, Nat. Chem. 2012, 4, 1011–1017;
- 2eR. E. Wilson, S. Skanthakumar, L. Soderholm, Angew. Chem. Int. Ed. 2011, 50, 11234–11237; Angew. Chem. 2011, 123, 11430–11433;
- 2fT. E. Albrecht-Schmitt, Angew. Chem. Int. Ed. 2005, 44, 4836–4838; Angew. Chem. 2005, 117, 4914–4916;
- 2gJ. D. Rinehart, S. A. Kozimor, J. R. Long, Angew. Chem. Int. Ed. 2010, 49, 2560–2564; Angew. Chem. 2010, 122, 2614–2618;
- 2hW. J. Evans, S. A. Kozimor, J. W. Ziller, Science 2005, 309, 1835–1838;
- 2iG. Nocton, J. Pecaut, M. Mazzanti, Angew. Chem. Int. Ed. 2008, 47, 3040–3042; Angew. Chem. 2008, 120, 3082–3084.
- 3
- 3aA. B. Kersting, D. W. Efurd, D. L. Finnegan, D. J. Rokop, D. K. Smith, J. L. Thompson, Nature 1999, 397, 56–59;
- 3bA. P. Novikov, S. N. Kalmykov, S. Utsunomiya, R. C. Ewing, F. Horreard, A. Merkulov, S. B. Clark, V. V. Tkachev, B. F. Myasoedov, Science 2006, 314, 638–641;
- 3cY. Suzuki, S. D. Kelly, K. M. Kemner, J. F. Banfield, Nature 2002, 419, 134–134;
- 3dL. R. Morss, N. M. Edelstein, J. Fuger, The Chemistry of the Actinide and Transactinide Elements, Springer, Dordrecht, 2006;
10.1007/1-4020-3598-5 Google Scholar
- 3eP. Crançon, E. Pili, L. Charlet, Sci. Total Environ. 2010, 408, 2118–2128;
- 3fG. J. Vazquez, C. J. Dodge, A. J. Francis, Inorg. Chem. 2009, 48, 9485–9490.
- 4
- 4aD. E. Latta, C. A. Gorski, M. I. Boyanov, E. J. O'Loughlin, K. M. Kemner, M. M. Scherer, Environ. Sci. Technol. 2012, 46, 778–786;
- 4bM. Stylo, N. Neubert, Y. H. Wang, N. Monga, S. J. Romaniello, S. Weyer, R. Bernier-Latmani, Proc. Natl. Acad. Sci. USA 2015, 112, 5619–5624.
- 5K. P. Carter, J. W. Jian, M. M. Pyrch, T. Z. Forbes, T. M. Eaton, R. J. Abergel, W. A. de Jong, J. K. Gibson, Chem. Commun. 2018, 54, 10698–10701.
- 6
- 6aS. Takao, K. Takao, W. Kraus, F. Ernmerling, A. C. Scheinost, G. Bernhard, C. Hennig, Eur. J. Inorg. Chem. 2009, 4771–4775;
- 6bC. Hennig, S. Takao, K. Takao, S. Weiss, W. Kraus, F. Emmerling, A. C. Scheinost, J. Chem. Soc. Dalton Trans. 2012, 41, 12818–12823;
- 6cL. S. Natrajan, A. N. Swinburne, M. B. Andrews, S. Randall, S. L. Heath, Coord. Chem. Rev. 2014, 266, 171–193;
- 6dK. Takao, S. Takao, A. C. Scheinost, G. Bernhard, C. Hennig, Inorg. Chem. 2012, 51, 1336–1344;
- 6eK. E. Knope, R. E. Wilson, M. Vasiliu, D. A. Dixon, L. Soderholm, Inorg. Chem. 2011, 50, 9696–9704;
- 6fK. E. Knope, M. Vasiliu, D. A. Dixon, L. Soderholm, Inorg. Chem. 2012, 51, 4239–4249;
- 6gL. Soderholm, P. M. Almond, S. Skanthakumar, R. E. Wilson, P. C. Burns, Angew. Chem. Int. Ed. 2008, 47, 298–302; Angew. Chem. 2008, 120, 304–308.
- 7
- 7aA. Kondinski, T. N. Parac-Vogt, Front. Chem. 2018, 6, 346;
- 7bA. Müller, P. Gouzerh, Chem. Soc. Rev. 2012, 41, 7431–7463;
- 7cA. Dolbecq, E. Dumas, C. R. Mayer, P. Mialane, Chem. Rev. 2010, 110, 6009–6048;
- 7dD. L. Long, R. Tsunashima, L. Cronin, Angew. Chem. Int. Ed. 2010, 49, 1736–1758; Angew. Chem. 2010, 122, 1780–1803.
- 8P. C. Burns, K. A. Kubatko, G. Sigmon, B. J. Fryer, J. E. Gagnon, M. R. Antonio, L. Soderholm, Angew. Chem. Int. Ed. 2005, 44, 2135–2139; Angew. Chem. 2005, 117, 2173–2177.
- 9P. B. Duval, C. J. Burns, D. L. Clark, D. E. Morris, B. L. Scott, J. D. Thompson, E. L. Werkema, L. Jia, R. A. Andersen, Angew. Chem. Int. Ed. 2001, 40, 3357–3361;
10.1002/1521-3773(20010917)40:18<3357::AID-ANIE3357>3.0.CO;2-C CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 3461–3465.
- 10
- 10aL. M. Mokry, N. S. Dean, C. J. Carrano, Angew. Chem. Int. Ed. Engl. 1996, 35, 1497–1498; Angew. Chem. 1996, 108, 1676–1677;
- 10bG. Nocton, F. Burdet, J. Pecaut, M. Mazzanti, Angew. Chem. Int. Ed. 2007, 46, 7574–7578; Angew. Chem. 2007, 119, 7718–7722;
- 10cG. Nocton, J. Pecaut, Y. Filinchuk, M. Mazzanti, Chem. Commun. 2010, 46, 2757–2759;
- 10dV. Mougel, B. Biswas, J. Pecaut, M. Mazzanti, Chem. Commun. 2010, 46, 8648–8650;
- 10eJ. C. Berthet, P. Thuery, M. Ephritikhine, Chem. Commun. 2005, 3415–3417;
- 10fJ. C. Berthet, P. Thuery, M. Ephritikhine, Inorg. Chem. 2010, 49, 8173–8177;
- 10gB. Biswas, V. Mougel, J. Pecaut, M. Mazzanti, Angew. Chem. Int. Ed. 2011, 50, 5745–5748; Angew. Chem. 2011, 123, 5863–5866;
- 10hC. Tamain, T. Dumas, C. Hennig, P. Guilbaud, Chem. Eur. J. 2017, 23, 6864–6875;
- 10iC. Falaise, H. A. Neal, M. Nyman, Inorg. Chem. 2017, 56, 6591–6598;
- 10jJ. Diwu, S. Wang, T. E. Albrecht-Schmitt, Inorg. Chem. 2012, 51, 4088–4093;
- 10kN. A. Vanagas, J. N. Wacker, C. L. Rom, E. N. Glass, I. Colliard, Y. S. Qiao, J. A. Bertke, E. Van Keuren, E. J. Schelter, M. Nyman, K. E. Knope, Inorg. Chem. 2018, 57, 7259–7269.
- 11
- 11aV. Mougel, P. Horeglad, G. Nocton, J. Pecaut, M. Mazzanti, Chem. Eur. J. 2010, 16, 14365–14377;
- 11bV. Mougel, J. Pecaut, M. Mazzanti, Chem. Commun. 2012, 48, 868–870.
- 12C. Falaise, C. Volkringer, J.-F. Vigier, A. Beaurain, P. Roussel, P. Rabu, T. Loiseau, J. Am. Chem. Soc. 2013, 135, 15678–15681.
- 13N. P. Martin, C. Volkringer, N. Henry, X. Trivelli, G. Stoclet, A. Ikeda-Ohno, T. Loiseau, Chem. Sci. 2018, 9, 5021–5032.
- 14Y. J. Hu, K. E. Knope, S. Skanthakumar, L. Soderholm, Eur. J. Inorg. Chem. 2013, 4159–4163.
- 15
- 15aI. D. Brown, D. Altermatt, Acta Crystallogr. Sect. B 1985, 41, 244–247;
- 15bP. C. Burns, R. C. Ewing, F. C. Hawthorne, Can. Mineral. 1997, 35, 1551–1570.
- 16A. E. Enriquez, B. L. Scott, M. P. Neu, Inorg. Chem. 2005, 44, 7403–7413.
- 17C. H. Zhan, J. M. Cameron, J. Gao, J. W. Purcell, D. L. Long, L. Cronin, Angew. Chem. Int. Ed. 2014, 53, 10362–10366; Angew. Chem. 2014, 126, 10530–10534.
- 18
- 18aR. D. Swartz, M. K. Coggins, W. Kaminsky, J. A. Kovacs, J. Am. Chem. Soc. 2011, 133, 3954–3963;
- 18bJ. H. Kim, J. Britten, J. Chin, J. Am. Chem. Soc. 1993, 115, 3618–3622;
- 18cR. Cini, F. P. Fanizzi, F. P. Intini, L. Maresca, G. Natile, J. Am. Chem. Soc. 1993, 115, 5123–5131.