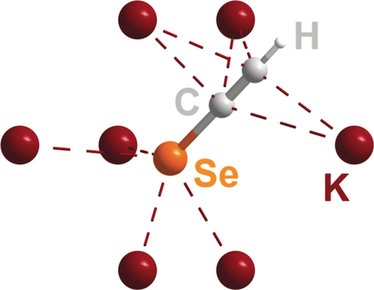
AISeC2H (AI=K, Rb, Cs): Crystalline Compounds with the Elusive −Se-C≡C-H Anion
Dedicated to Professor Alexander C. Filippou on the occasion of his 60th birthday
Graphical Abstract
Abstract
By reaction of alkali metal acetylides, AIC2H (AI=K, Rb, Cs), with elemental selenium in liquid ammonia highly crystalline powders of AISeC2H were obtained. The structure analysis based on the resulting synchrotron powder diffraction data revealed that all compounds crystallize in an orthorhombic unit cell (Cmc21, Z=4) exhibiting the elusive −SeC2H anion, which is unprecedented in a crystalline compound up to now. Elemental analysis and IR spectroscopic data confirm this finding. Upon heating, AISeC2H compounds release acetylene based on DSC/TGA experiments resulting in powders with the proposed composition AI2Se2(C2). The resulting powders were indexed with small cubic unit cells, but a reasonable structural model could not be developed up to now. Upon exposure of AISeC2H compounds to water elemental selenium is formed again.