Wavelength-Controlled Dynamic Metathesis: A Light-Driven Exchange Reaction between Disulfide and Diselenide Bonds
Fuqiang Fan
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
College of Sciences, Northeastern University, Shenyang, 110819 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Shaobo Ji
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorChenxing Sun
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorCheng Liu
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorDr. Ying Yu
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorProf. Yu Fu
College of Sciences, Northeastern University, Shenyang, 110819 China
Search for more papers by this authorCorresponding Author
Prof. Huaping Xu
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorFuqiang Fan
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
College of Sciences, Northeastern University, Shenyang, 110819 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Shaobo Ji
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorChenxing Sun
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorCheng Liu
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorDr. Ying Yu
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorProf. Yu Fu
College of Sciences, Northeastern University, Shenyang, 110819 China
Search for more papers by this authorCorresponding Author
Prof. Huaping Xu
Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorGraphical Abstract
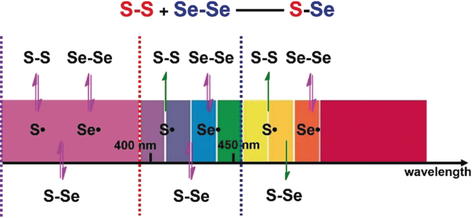
Controlled metathesis: Metathesis between disulfide and diselenide bonds was realized under irradiation, and the conversion of the exchange reaction could be controlled by modulating the wavelength of the light. This chemistry was applied to polymer materials to control the cleavage of polymers from a distance.
Abstract
Wavelength-controlled dynamic processes are mostly based on light-triggered isomerization or the cleavage/formation of molecular connections. Control over dynamic metathesis reactions by different light wavelengths, which would be useful in controllable dynamic chemistry, has rarely been studied. Taking advantage of the different bond energies of disulfide and diselenide bonds, we have developed a wavelength-driven exchange reaction between disulfides and diselenides, which underwent metathesis under UV light to produce Se−S bonds. When irradiated with visible light, the Se−S bonds were reversed back to those of the original reactants. The conversion of the exchange depends on the wavelength of the incident light. This light-driven metathesis chemistry was also applied to tune the mechanical properties of polymer materials. The visible-light-induced reverse reaction was compatible with reductant-catalyzed disulfide/diselenide metathesis, and could be utilized to develop a dissipative system with light as the energy input.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810297-sup-0001-misc_information.pdf1,020.1 KB | Supplementary |
| anie201810297-sup-0001-VideoS1.mp44.9 MB | Supplementary |
| anie201810297-sup-0001-VideoS2.mp41.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. M. Lehn, Chem. Eur. J. 1999, 5, 2455–2463.
10.1002/(SICI)1521-3765(19990903)5:9<2455::AID-CHEM2455>3.0.CO;2-H CAS Web of Science® Google Scholar
- 2S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders, J. F. Stoddart, Angew. Chem. Int. Ed. 2002, 41, 898–952; Angew. Chem. 2002, 114, 938–993.
- 3P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J. L. Wietor, J. K. M. Sanders, S. Otto, Chem. Rev. 2006, 106, 3652–3711.
- 4Y. Jin, C. Yu, R. J. Denman, W. Zhang, Chem. Soc. Rev. 2013, 42, 6634–6654.
- 5C. B. Minkenberg, L. Florusse, R. Eelkema, G. J. Koper, J. H. van Esch, J. Am. Chem. Soc. 2009, 131, 11274–11275.
- 6C. B. Minkenberg, F. Li, P. van Rijn, L. Florusse, J. Boekhoven, M. C. A. Stuart, G. J. M. Koper, R. Eelkema, J. H. van Esch, Angew. Chem. Int. Ed. 2011, 50, 3421–3424; Angew. Chem. 2011, 123, 3483–3486.
- 7K. D. Okochi, G. S. Han, I. M. Aldridge, Y. Liu, W. Zhang, Org. Lett. 2013, 15, 4296–4299.
- 8M. Mondal, N. Radeva, H. Koster, A. Park, C. Potamitis, M. Zervou, G. Klebe, A. K. H. Hirsch, Angew. Chem. Int. Ed. 2014, 53, 3259–3263; Angew. Chem. 2014, 126, 3324–3328.
- 9B. T. Michal, C. A. Jaye, E. J. Spencer, S. J. Rowan, ACS Macro Lett. 2013, 2, 694–699.
- 10P. Taynton, H. Ni, C. Zhu, K. Yu, S. Loob, Y. Jin, H. Qi, W. Zhang, Adv. Mater. 2016, 28, 2904–2909.
- 11M. Röttger, T. Domenech, R. van der Weegen, A. Breuillac, R. Nicolay, L. Leibler, Science 2017, 356, 62–65.
- 12G. D. Bo, Chem 2016, 1, 668–673.
- 13M. Nakahata, S. Mori, T. Takashima, H. Yamaguchi, A. Harada, Chem 2016, 1, 766–775.
- 14P. Reutenauer, P. J. Boul, J. M. Lehn, Eur. J. Org. Chem. 2009, 1691–1697.
- 15R. C. Boutelle, B. H. J. Northrop, Org. Chem. 2011, 76, 7994–8002.
- 16M. Capela, N. J. Mosey, L. Xing, R. Wang, A. Petitjean, Chem. Eur. J. 2011, 17, 4598–4612.
- 17M. E. Belowich, J. F. Stoddart, Chem. Soc. Rev. 2012, 41, 2003–2024.
- 18Y. Yi, H. Xu, L. Wang, W. Cao, X. Zhang, Chem. Eur. J. 2013, 19, 9506–9510.
- 19J. Li, J. M. Carnall, M. C. Stuart, S. Otto, Angew. Chem. Int. Ed. 2011, 50, 8384–8386; Angew. Chem. 2011, 123, 8534–8536.
- 20S. Ji, W. Cao, Y. Yu, H. Xu, Angew. Chem. Int. Ed. 2014, 53, 6781–6785; Angew. Chem. 2014, 126, 6899–6903.
- 21M. J. Hansen, W. A. Velema, M. M. Lerch, W. Szymanski, B. L. Feringa, Chem. Soc. Rev. 2015, 44, 3358–3377.
- 22M. Kathan, S. Hecht, Chem. Soc. Rev. 2017, 46, 5536–5550.
- 23H. Frisch, D. E. Marschner, A. S. Goldmann, C. Barner-Kowollik, Angew. Chem. Int. Ed. 2018, 57, 2036–2045; Angew. Chem. 2018, 130, 2054–2064.
- 24M. Herder, J. M. Lehn, J. Am. Chem. Soc. 2018, 140, 7647–7657.
- 25G. S. Hartley, Nature 1937, 140, 281–281.
- 26H. M. Bandara, S. C. Burdette, Chem. Soc. Rev. 2012, 41, 1809–1825.
- 27J. W. Fredy, A. Mendez-Ardoy, S. Kwangmettatam, D. Bochicchio, B. Matt, M. C. A. Stuart, J. Huskens, N. Katsonis, G. M. Pavan, T. Kudernac, Proc. Natl. Acad. Sci. USA 2017, 114, 11850–11855.
- 28H. Huang, A. Juan, N. Katsonis, J. Huskens, Tetrahedron 2017, 73, 4913–4917.
- 29R. Klajn, Chem. Soc. Rev. 2014, 43, 148–184.
- 30M. Irie, Chem. Rev. 2000, 100, 1685–1716.
- 31M. Irie, T. Fukaminato, K. Matsuda, S. Kobatake, Chem. Rev. 2014, 114, 12174–12277.
- 32M. Irie, M. J. Mohri, Org. Chem. 1988, 53, 803–808.
- 33J. J. de Jong, P. R. Hania, A. Pugzlys, L. N. Lucas, M. de Loos, R. M. Kellogg, B. L. Feringa, K. Duppen, J. H. van Esch, Angew. Chem. Int. Ed. 2005, 44, 2373–2376; Angew. Chem. 2005, 117, 2425–2428.
- 34A. Fürstner, Angew. Chem. Int. Ed. 2000, 39, 3012–3043;
10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 3140–3172.
- 35S. T. Nguyen, L. K. Johnson, R. H. Grubbs, J. W. Ziller, J. Am. Chem. Soc. 1992, 114, 3974–3975.
- 36A. G. Vandeputte, M. K. Sabbe, M. F. Reyniers, G. B. Marin, Chem. Eur. J. 2011, 17, 7656–7673.
- 37M. Kathan, F. Eisenreich, C. Jurssek, A. Dallmann, J. Gurke, S. Hecht, Nat. Chem. 2018, 10, 1031–1036.
- 38S. Ji, J. Xia, H. Xu, ACS Macro Lett. 2016, 5, 78–82.
- 39S. Ji, W. Cao, Y. Yu, H. Xu, Adv. Mater. 2015, 27, 7740–7745.
- 40J. Xia, S. Ji, H. Xu, Polym. Chem. 2016, 7, 6708–6713.
- 41S. Ji, H. El Mard, M. Smet, W. Dehaen, H. Xu, Sci. China Chem. 2017, 60, 1191–1196.
- 42S. Ji, F. Fan, C. Sun, Y. Yu, H. Xu, ACS Appl. Mater. Interfaces 2017, 9, 33169–33175.
- 43S. Otto, R. L. E. Furlan, J. K. M. Sanders, J. Am. Chem. Soc. 2000, 122, 12063–12064.
- 44O. Ramström, J. M. Lehn, ChemBioChem 2000, 1, 41–48.
- 45N. Zhu, F. Zhang, G. J. Liu, Comb. Chem. 2010, 12, 531–540.
- 46J. W. Sadownik, E. Mattia, P. Nowak, S. Otto, Nat. Chem. 2016, 8, 264–269.
- 47B. M. Matysiak, P. Nowak, I. Cvtila, C. G. Pappas, B. Liu, D. Komaromy, S. Otto, J. Am. Chem. Soc. 2017, 139, 6744–6751.
- 48H. Xu, W. Cao, X. Zhang, Acc. Chem. Res. 2013, 46, 1647–1658.
- 49W. Cao, X. Zhang, X. Miao, Z. Yang, H. Xu, Angew. Chem. Int. Ed. 2013, 52, 6233–6237; Angew. Chem. 2013, 125, 6353–6357.
- 50W. Cao, L. Wang, H. Xu, Nano Today 2015, 10, 717–736.
- 51Y. Zhao, D. G. Truhlar, Acc. Chem. Res. 2008, 41, 157–167.
- 52M. O. Andreae, P. J. Crutzen, Science 1997, 276, 1052–1058.
- 53N. K. J. Kildahl, J. Chem. Educ. 1995, 72, 0.
- 54E. S. J. Arnér, E. J. Holmgren, Biochemistry 2000, 267, 6102–6109.
- 55R. J. Singh, N. Hogg, J. Joseph, B. Kalyanaraman, J. Biol. Chem. 1996, 271, 18596–18603.