The Chemical Properties of Hydrogen Atoms Adsorbed on M0-Nanoparticles Suspended in Aqueous Solutions: The Case of Ag0-NPs and Au0-NPs Reduced by BD4−
Alina Sermiagin
Chemical Sciences Department, Ariel University, Kyriat Hamada 3, Ariel, 40700 Israel
Search for more papers by this authorProf. Dr. Dan Meyerstein
Chemical Sciences Department, Ariel University, Kyriat Hamada 3, Ariel, 40700 Israel
Chemistry Department, Ben-Gurion University of the Negev, Beer-Sheva, 84105 Israel
Search for more papers by this authorDr. Ronen Bar-Ziv
Chemistry Department, Nuclear Research Centre Negev, Beer-Sheva, 84190 Israel
Search for more papers by this authorCorresponding Author
Dr. Tomer Zidki
Chemical Sciences Department, Ariel University, Kyriat Hamada 3, Ariel, 40700 Israel
Search for more papers by this authorAlina Sermiagin
Chemical Sciences Department, Ariel University, Kyriat Hamada 3, Ariel, 40700 Israel
Search for more papers by this authorProf. Dr. Dan Meyerstein
Chemical Sciences Department, Ariel University, Kyriat Hamada 3, Ariel, 40700 Israel
Chemistry Department, Ben-Gurion University of the Negev, Beer-Sheva, 84105 Israel
Search for more papers by this authorDr. Ronen Bar-Ziv
Chemistry Department, Nuclear Research Centre Negev, Beer-Sheva, 84190 Israel
Search for more papers by this authorCorresponding Author
Dr. Tomer Zidki
Chemical Sciences Department, Ariel University, Kyriat Hamada 3, Ariel, 40700 Israel
Search for more papers by this authorGraphical Abstract
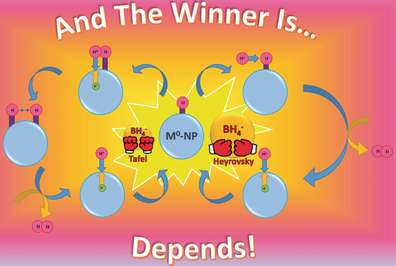
The nature of H-atoms adsorbed on metal nanoparticles (NPs) is of major importance in many catalyzed reduction processes. The H-atoms of the transient {(M0-NP)-Hn}n− behave mainly as hydrides when n is large (the Heyrovsky mechanism), and as atoms when n is small (the Tafel mechanism). The relative contributions of the two mechanisms differ considerably for Au and Ag-NPs.
Abstract
The nature of H-atoms adsorbed on M0-nanoparticles is of major importance in many catalyzed reduction processes. Using isotope labeling, we determined that hydrogen evolution from transient {(M0-NP)-Hn}n− proceeds mainly via the Heyrovsky mechanism when n is large (i.e., the hydrogens behave as hydrides) but mainly via the Tafel mechanism when n is small (i.e., the hydrogens behave as atoms). Additionally, the relative contributions of the two mechanisms differ considerably for M=Au and Ag. The results are analogous to those recently reported for the M0-NP-catalyzed de-halogenation processes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809302-sup-0001-misc_information.pdf596.9 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Heyes, M. Dunwell, B. Xu, J. Phys. Chem. C 2016, 120, 17334–17341.
- 2A. R. Kucernak, C. Zalitis, J. Phys. Chem. C 2016, 120, 10721–10745.
- 3D. Duan, H. Liu, Q. Wang, Y. Wang, S. Liu, Electrochim. Acta 2016, 198, 212–219.
- 4M. Grdeń, M. Łukaszewski, G. Jerkiewicz, A. Czerwiński, Electrochim. Acta 2008, 53, 7583–7598.
- 5J. W. Lee, S. Il Pyun, Electrochim. Acta 2005, 50, 1777–1805.
- 6J. D. Goodpaster, A. T. Bell, M. Head-Gordon, J. Phys. Chem. Lett. 2016, 7, 1471–1477.
- 7P. S. Maiti, A. K. Ganai, R. Bar-Ziv, A. N. Enyashin, L. Houben, M. Bar Sadan, Chem. Mater. 2018, 30, 4489–4492.
- 8T. Grewe, M. Meggouh, H. Tüysüz, Chem. Asian J. 2016, 11, 22–42.
- 9A. V. Puga, Coord. Chem. Rev. 2016, 315, 1–66.
- 10K. Zhang, L. Guo, Catal. Sci. Technol. 2013, 3, 1672–1690.
- 11R. Marcos, L. Xue, R. Sánchez-De-Armas, M. S. G. Ahlquist, ACS Catal. 2016, 6, 2923–2929.
- 12A. M. Kalekar, K. K. K. Sharma, M. N. Luwang, G. K. Sharma, RSC Adv. 2016, 6, 11911–11920.
- 13B. Sun, D. Carnevale, G. Süss-Fink, J. Organomet. Chem. 2016, 821, 197–205.
- 14J. Mondal, S. K. Kundu, W. K. Hung Ng, R. Singuru, P. Borah, H. Hirao, Y. Zhao, A. Bhaumik, Chem. Eur. J. 2015, 21, 19016–19027.
- 15A. Noschese, A. Buonerba, P. Canton, S. Milione, C. Capacchione, A. Grassi, J. Catal. 2016, 340, 30–40.
- 16S. Anantharaj, M. Jayachandran, S. Kundu, Chem. Sci. 2016, 7, 3188–3205.
- 17A. K. Sasmal, S. Dutta, T. Pal, Dalton Trans. 2016, 45, 3139–3150.
- 18P. Zhao, X. Feng, D. Huang, G. Yang, D. Astruc, Coord. Chem. Rev. 2015, 287, 114–136.
- 19S. E. Kudaibergenov, G. S. Tatykhanova, B. S. Selenova, J. Inorg. Organomet. Polym. Mater. 2016, 26, 1198–1211.
- 20J. Hwang, A. B. Siddique, Y. J. Kim, H. Lee, J. H. Maeng, Y. Ahn, J. S. Lee, H. S. Kim, H. Lee, RSC Adv. 2018, 8, 1758–1763.
- 21T. Parandhaman, N. Pentela, B. Ramalingam, D. Samanta, S. K. Das, ACS Sustainable Chem. Eng. 2017, 5, 489–501.
- 22N. Chavda, A. Trivedi, J. Thakarda, Y. K. Agrawal, P. Maity, Catal. Lett. 2016, 146, 1331–1339.
- 23K. Kaneda, T. Mitsudome, Chem. Rec. 2017, 17, 4–26.
- 24G. Merga, R. Wilson, G. Lynn, B. H. Milosavljevic, D. Meisel, J. Phys. Chem. C 2007, 111, 12220–12226.
- 25G. Mills, A. Henglein, Rad. Chem. Phys. 1985, 26, 385–390.
- 26H. H. Shin, L. Lu, Z. Yang, C. J. Kiely, S. McIntosh, ACS Catal. 2016, 6, 2811–2818.
- 27B. G. Ershov, E. V. Abkhalimov, R. D. Solovov, V. I. Roldughin, Phys. Chem. Chem. Phys. 2016, 18, 13459–13466.
- 28E. M. Kumar, B. Prajapat, B. Saha, R. Thapa, Int. J. Hydrogen Energy 2016, 41, 3928–3939.
- 29L. Wang, C. Yin, R. T. Yang, Appl. Catal. A 2016, 514, 35–42.
- 30M. Filez, E. A. Redekop, V. V. Galvita, H. Poelman, M. Meledina, S. Turner, G. Van Tendeloo, A. T. Bell, G. B. Marin, Phys. Chem. Chem. Phys. 2016, 18, 3234–3243.
- 31S. K. Konda, A. Chen, Mater. Today 2016, 19, 100–108.
- 32H. Cheng, L. Chen, A. C. Cooper, X. Sha, G. P. Pez, G. P. Pez, M. Roth, M. Sing, M. Schmidt, E. Sohmen, Energy Environ. Sci. 2008, 1, 338–354.
- 33H. Shin, M. Choi, H. Kim, Y. R. Shen, R. F. Lobo, U. Nagashima, H. Ogawa, T. Kyotani, M. Ito, Phys. Chem. Chem. Phys. 2016, 18, 7035–7041.
- 34F. R. Lucci, M. D. Marcinkowski, T. J. Lawton, E. C. H. Sykes, J. Phys. Chem. C 2015, 119, 24351–24357.
- 35C. Lin, T. Xu, J. Yu, Q. Ge, Z. Xiao, J. Phys. Chem. C 2009, 113, 8513–8517.
- 36R. Roszak, L. Firlej, S. Roszak, P. Pfeifer, B. Kuchta, Colloids Surf. A 2016, 496, 69–76.
- 37M. Yamauchi, H. Kobayashi, H. Kitagawa, ChemPhysChem 2009, 10, 2566–2576.
- 38S. H. Mushrif, A. D. Rey, G. H. Peslherbe, J. Mater. Chem. 2010, 20, 10503–10510.
- 39M. S. Matheson, P. C. Lee, D. Meisel, E. Pelizzetti, J. Phys. Chem. 1983, 87, 394–399.
- 40D. Meisel, W. A. Mulac, M. S. Matheson, J. Phys. Chem. 1981, 85, 179–187.
- 41S. Haghighat, J. M. Dawlaty, J. Phys. Chem. C 2016, 120, 28489–28496.
- 42R. Ishida, S. Hayashi, S. Yamazoe, K. Kato, T. Tsukuda, J. Phys. Chem. Lett. 2017, 8, 2368–2372.
- 43K. A. Holbrook, P. J. Twist, J. Chem. Soc. A 1971, 890–894.
- 44G. Guella, B. Patton, A. Miotello, J. Phys. Chem. C 2007, 111, 18744–18750.
- 45G. Guella, C. Zanchetta, B. Patton, A. Miotello, J. Phys. Chem. B 2006, 110, 17024–17033.
- 46R. Bar-Ziv, I. Zilbermann, O. Oster-Golberg, T. Zidki, G. Yardeni, H. Cohen, D. Meyerstein, Chem. Eur. J. 2012, 18, 4699–4705.
- 47J. Adhikary, D. Meyerstein, V. Marks, M. Meistelman, G. Gershinsky, A. Burg, D. Shamir, H. Kornweitz, Y. Albo, Appl. Catal. B 2018, 239, 450–462.
- 48J. Adhikary, M. Meistelman, A. Burg, D. Shamir, D. Meyerstein, Y. Albo, Eur. J. Inorg. Chem. 2017, 1510–1515.
- 49M. M. Kreevoy, J. E. C. Hutchins, J. Am. Chem. Soc. 1972, 94, 6371–6376.
- 50I. Roger, M. A. Shipman, M. D. Symes, Nat. Rev. Chem. 2017, https://doi.org/10.1038/s41570-016-0003.
- 51S. Harinipriya, M. V. Sangaranarayanan, J. Phys. Chem. B 2002, 106, 8681–8688.