Propagation of Oscillating Chemical Signals through Reaction Networks
Dr. Rafał Roszak
Institute of Organic Chemistry, Polish Academy of Sciences, Ul. Kasprzaka 44/52, Warsaw, 02-224 Poland
Search for more papers by this authorMichał D. Bajczyk
Institute of Organic Chemistry, Polish Academy of Sciences, Ul. Kasprzaka 44/52, Warsaw, 02-224 Poland
Search for more papers by this authorEwa P. Gajewska
Institute of Organic Chemistry, Polish Academy of Sciences, Ul. Kasprzaka 44/52, Warsaw, 02-224 Poland
Search for more papers by this authorCorresponding Author
Prof. Robert Hołyst
Institute of Physical Chemistry, Polish Academy of Sciences, Ul. Kasprzaka 44/52, Warsaw, 02-224 Poland
Search for more papers by this authorCorresponding Author
Prof. Bartosz A. Grzybowski
Institute of Organic Chemistry, Polish Academy of Sciences, Ul. Kasprzaka 44/52, Warsaw, 02-224 Poland
IBS Center for Soft and Living Matter and Department of Chemistry, UNIST, 50, UNIST-gil, Eonyang-eup, Ulju-gun, Ulsan, South Korea
Search for more papers by this authorDr. Rafał Roszak
Institute of Organic Chemistry, Polish Academy of Sciences, Ul. Kasprzaka 44/52, Warsaw, 02-224 Poland
Search for more papers by this authorMichał D. Bajczyk
Institute of Organic Chemistry, Polish Academy of Sciences, Ul. Kasprzaka 44/52, Warsaw, 02-224 Poland
Search for more papers by this authorEwa P. Gajewska
Institute of Organic Chemistry, Polish Academy of Sciences, Ul. Kasprzaka 44/52, Warsaw, 02-224 Poland
Search for more papers by this authorCorresponding Author
Prof. Robert Hołyst
Institute of Physical Chemistry, Polish Academy of Sciences, Ul. Kasprzaka 44/52, Warsaw, 02-224 Poland
Search for more papers by this authorCorresponding Author
Prof. Bartosz A. Grzybowski
Institute of Organic Chemistry, Polish Academy of Sciences, Ul. Kasprzaka 44/52, Warsaw, 02-224 Poland
IBS Center for Soft and Living Matter and Department of Chemistry, UNIST, 50, UNIST-gil, Eonyang-eup, Ulju-gun, Ulsan, South Korea
Search for more papers by this authorGraphical Abstract
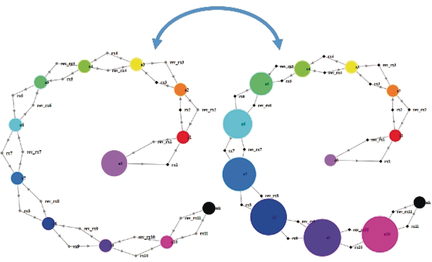
Reaction networks do not like high frequencies: Similar to electronic systems that can tune to and process signals of select frequencies, systems/networks of chemical reactions “propagate” oscillatory concentration inputs in a frequency-dependent manner. In particular, simulations in the Kinetix software reveal that for diverse system architectures oscillations are transmitted only up to a certain threshold value and are dampened for higher frequencies.
Abstract
Akin to electronic systems that can tune to and process signals of select frequencies, systems/networks of chemical reactions also “propagate” time-varying concentration inputs in a frequency-dependent manner. Whereas signals of low frequencies are transmitted, higher frequency inputs are dampened and converted into steady-concentration outputs. Such behavior is observed in both idealized reaction chains as well as realistic signaling cascades, in the latter case explaining the experimentally observed responses of such cascades to input calcium oscillations. These and other results are supported by numerical simulations within the freely available Kinetix web application we developed to study chemical systems of arbitrary architectures, reaction kinetics, and boundary conditions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201808821-sup-0001-misc_information.pdf1.7 MB | Supplementary |
| anie201808821-sup-0001-Movie_S1.mp49.4 MB | Supplementary |
| anie201808821-sup-0001-Movie_S2.mp45.1 MB | Supplementary |
| anie201808821-sup-0001-Movie_S3.mp47.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. A. Papin, N. D. Price, S. J. Wiback, D. A. Fell, B. O. Palsson, Trends Biochem. Sci. 2003, 28, 250–258.
- 2
- 2aM. A. Fischbach, C. T. Walsh, Chem. Rev. 2006, 106, 3468–3496;
- 2bA. S. Khalil, J. J. Collins, Nat. Rev. Genet. 2010, 11, 367–379.
- 3G. Karlebach, R. Shamir, Nat. Rev. Mol. Cell Biol. 2008, 9, 770–780.
- 4
- 4aU. S. Bhalla, R. Iyengar, Science 1999, 283, 381–387;
- 4bN. Barkai, S. Leibler, Nature 1997, 387, 913–917.
- 5
- 5aR. F. Ludlow, S. Otto, Chem. Soc. Rev. 2008, 37, 101–108;
- 5bJ. Li, P. Nowak, S. Otto, J. Am. Chem. Soc. 2013, 135, 9222–9239;
- 5cE. Mattia, S. Otto, Nat. Nanotechnol. 2015, 10, 111–119;
- 5dB. A. Grzybowski, W. T. S. Huck, Nat. Nanotechnol. 2016, 11, 585–592;
- 5eB. A. Grzybowski, K. Fitzner, J. Paczesny, S. Granick, Chem. Soc. Rev. 2017, 46, 5647–5678.
- 6C. M. Gothard, S. Soh, N. A. Gothard, B. Kowalczyk, Y. Wei, B. Baytekin, B. A. Grzybowski, Angew. Chem. Int. Ed. 2012, 51, 7922–7927; Angew. Chem. 2012, 124, 8046–8051.
- 7
- 7aS. N. Semenov, A. J. Markvoort, T. F. A. de Greef, W. T. S. Huck, Angew. Chem. Int. Ed. 2014, 53, 8066–8069; Angew. Chem. 2014, 126, 8204–8207;
- 7bM. L. van Poll, F. Zhou, M. Ramstedt, L. Hu, W. T. S. Huck, Angew. Chem. Int. Ed. 2007, 46, 6634–6637; Angew. Chem. 2007, 119, 6754–6757.
- 8
- 8aM. Colomb-Delsuc, E. Mattia, J. W. Sadownik, S. Otto, Nat. Commun. 2015, 6, 7427;
- 8bS. Otto, Acc. Chem. Res. 2012, 45, 2200–2210;
- 8cA. Vidonne, D. Philp, Eur. J. Org. Chem. 2009, 593–610.
- 9
- 9aS. N. Semenov, A. S. Y. Wong, R. M. van der Made, S. G. J. Postma, J. Groen, H. W. H. van Roekel, T. F. A. de Greef, W. T. S. Huck, Nat. Chem. 2015, 7, 160–165;
- 9bS. N. Semenov, L. J. Kraft, A. Ainla, M. Zhao, M. Baghbanzadeh, V. E. Campbell, K. Kang, J. M. Fox, G. M. Whitesides, Nature 2016, 537, 656–660.
- 10J. Boekhoven, W. E. Hendriksen, G. J. M. Koper, R. Eelkema, J. H. van Esch, Science 2015, 349, 1075–1079.
- 11S. Dhiman, A. Jain, S. J. George, Angew. Chem. Int. Ed. 2017, 56, 1329–1333; Angew. Chem. 2017, 129, 1349–1353.
- 12
- 12aM. Samoilov, A. Arkin, J. Ross, J. Phys. Chem. A 2002, 106, 10205–10221;
- 12bS. Pramanik, I. Aprahamian, J. Am. Chem. Soc. 2016, 138, 15142–15145.
- 13J. J. Armao IV, J.-M. Lehn, J. Am. Chem. Soc. 2016, 138, 16809–16814.
- 14
- 14aWolfram Research, Inc., Mathematica, Version 11.1, Champaign, IL (2017);
- 14bMathworks, Inc., MATLAB release 2017a, Natick, Massachusetts (2017).
- 15The existing systems biology platforms (e.g., COPASI,[16a] Dizzy,[16b] GNA,[16c] or Omix[16d]) are not only based on relatively outdated, non-web technology (early 2000’s) and quite cumbersome to set up models, but they also do not allow to specify input signals as functions of time, which is the main feature of our current work.
- 16
- 16aS. Hoops, S. Sahle, R. Gauges, C. Lee, J. Pahle, N. Simus, M. Singhal, L. Xu, P. Mendes, U. Kummer, Bioinformatics 2006, 22, 3067–3074;
- 16bS. Ramsey, D. Orrell, H. Bolouri, J. Bioinf. Comput. Biol. 2005, 3, 415–436;
- 16cH. de Jong, J. Geiselmann, C. Hernandez, M. Page, Bioinformatics 2003, 19, 336–344;
- 16dP. Droste, S. Miebach, S. Niedenführ, W. Wiechert, K. Nöh, Biosystems 2011, 105, 154–161.
- 17For example, in a reaction A + B→C, both A and B participate in the reaction “operation”. Without the reaction node, one would have to draw arrows from A to B and from B to C. This would imply some nonsensical relationships, e.g., implying that a large product C is made from a small reagent B. For detailed discussion bipartite reaction networks, see Ref. [18].
- 18
- 18aS. Szymkuć, E. P. Gajewska, T. Klucznik, K. Molga, P. Dittwald, M. Startek, M. Bajczyk, B. A. Grzybowski, Angew. Chem. Int. Ed. 2016, 55, 5904–5937; Angew. Chem. 2016, 128, 6004–6040;
- 18bM. Kowalik, C. M. Gothard, A. M. Drews, N. A. Gothard, A. Wieckiewicz, P. E. Fuller, B. A. Grzybowski, K. J. M. Bishop, Angew. Chem. Int. Ed. 2012, 51, 7928–7932; Angew. Chem. 2012, 124, 8052–8056;
- 18cT. Klucznik, B. Mikulak-Klucznik, M. P. McCormack, H. Lima, S. Szymkuć, M. Bhowmick, K. Molga, Y. Zhou, L. Rickershauser, E. P. Gajewska, A. Toutchkine, P. Dittwald, M. P. Startek, G. J. Kirkovits, R. Roszak, A. Adamski, B. Sieredzińska, M. Mrksich, S. L. J. Trice, B. A. Grzybowski, Chem 2018, 4, 522–532.
- 19Analytical solution for a chain of n reversible reactions, Ai−1⇌Ai, is somewhat cumbersome since all reactions steps are coupled along the chain—in effect, to find eigenvectors and eigenvalues, it is necessary to first diagonalize a n x n matrix. One case that is relatively easy to solve is when only the first reaction is reversible and the following ones are irreversible, Ao→A1→A2→…. For the first case, the amplitudes at the first two nodes are related by A1/A0=
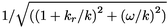 —as seen, as kr increases, A1 decreases.
—as seen, as kr increases, A1 decreases.
- 20
- 20aC. J. Marshall, Cell 1995, 80, 179–185;
- 20bL. Chang, M. Karin, Nature 2001, 410, 37–40;
- 20cJ. A. McCubrey, L. S. Steelman, W. H. Chappell, S. L. Abrams, E. W. T. Wong, F. Chang, B. Lehmann, D. M. Terrian, M. Milella, A. Tafuri, F. Stivala, M. Libra, J. Basecke, C. Evangelisti, A. M. Martelli, R. A. Franklin, Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1263–1284.
- 21
- 21aB. N. Kholodenko, J. F. Hancock, W. Kolch, Nat. Rev. Mol. Cell Biol. 2010, 11, 414–426;
- 21bM. Yi, Q. Zhao, J. Tang, C. Wang, Biophys. Chem. 2011, 157, 33–42;
- 21cB. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts, P. Walter, Molecular Biology of the Cell, 5th ed., American Society for Cell Biology, New York, 2008.
- 22
- 22aE. Smedler, P. Uhlén, Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 964–969;
- 22bM. J. Boulware, J. S. Marchant, Curr. Biol. 2008, 18, R 769–R776;
- 22cP. J. Cullen, P. J. Lockyer, Nat. Rev. Mol. Cell Biol. 2002, 3, 339–348.
- 23S. Kupzig, S. A. Walker, P. J. Cullen, Proc. Natl. Acad. Sci. USA 2005, 102, 7577–7582.
- 24
- 24ahttps://www.meteor.com/;
- 24bhttps://d3js.org/;
- 24chttps://www.mongodb.com/.
- 25
- 25aE. Hairer, G. Wanner, Solving Ordinary Differential Equations II. Stiff and Differential-Algebraic Problems, Springer, Berlin, 1991;
10.1007/978-3-662-09947-6 Google Scholar
- 25bE. Süli, D. F. Mayers, An Introduction to Numerical Analysis, Cambridge University Press, Cambridge, 2003;
10.1017/CBO9780511801181 Google Scholar
- 25cJ. Crank, P. Nicolson, Math. Proc. Cambridge Philos. Soc. 1947, 43, 50–67.