Air-Stable Lithium Spheres Produced by Electrochemical Plating
Dr. Tingting Yang
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
These authors contributed equally to this work.
Search for more papers by this authorPeng Jia
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
These authors contributed equally to this work.
Search for more papers by this authorQiunan Liu
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Dr. Liqiang Zhang
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
State Key Laboratory of Heavy Oil Processing, Beijing Key Laboratory of Failure, Corrosion, and Protection of Oil/Gas Facilities, China University of Petroleum Beijing, Beijing, 102249 China
Search for more papers by this authorCongcong Du
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
These authors contributed equally to this work.
Search for more papers by this authorJingzhao Chen
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorHongjun Ye
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorXiaomei Li
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorYanshuai Li
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorCorresponding Author
Prof. Tongde Shen
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorCorresponding Author
Dr. Yongfu Tang
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Hebei Key Laboratory of Applied Chemistry, College of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorCorresponding Author
Dr. Jianyu Huang
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Key Laboratory of Low Dimensional Materials and Application Technology of Ministry of Education, School of Materials Science and Engineering, Xiangtan University, Xiangtan, Hunan, 411105 China
Search for more papers by this authorDr. Tingting Yang
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
These authors contributed equally to this work.
Search for more papers by this authorPeng Jia
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
These authors contributed equally to this work.
Search for more papers by this authorQiunan Liu
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Dr. Liqiang Zhang
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
State Key Laboratory of Heavy Oil Processing, Beijing Key Laboratory of Failure, Corrosion, and Protection of Oil/Gas Facilities, China University of Petroleum Beijing, Beijing, 102249 China
Search for more papers by this authorCongcong Du
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
These authors contributed equally to this work.
Search for more papers by this authorJingzhao Chen
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorHongjun Ye
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorXiaomei Li
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorYanshuai Li
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorCorresponding Author
Prof. Tongde Shen
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorCorresponding Author
Dr. Yongfu Tang
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Hebei Key Laboratory of Applied Chemistry, College of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao, 066004 China
Search for more papers by this authorCorresponding Author
Dr. Jianyu Huang
Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, 066004 China
Key Laboratory of Low Dimensional Materials and Application Technology of Ministry of Education, School of Materials Science and Engineering, Xiangtan University, Xiangtan, Hunan, 411105 China
Search for more papers by this authorGraphical Abstract
Abstract
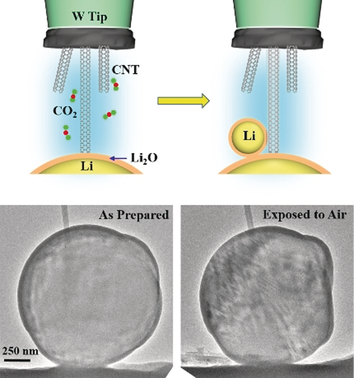
Lithium metal is an ideal anode for next-generation lithium batteries owing to its very high theoretical specific capacity of 3860 mAh g−1 but very reactive upon exposure to ambient air, rendering it difficult to handle and transport. Air-stable lithium spheres (ASLSs) were produced by electrochemical plating under CO2 atmosphere inside an advanced aberration-corrected environmental transmission electron microscope. The ASLSs exhibit a core–shell structure with a Li core and a Li2CO3 shell. In ambient air, the ASLSs do not react with moisture and maintain their core–shell structures. Furthermore, the ASLSs can be used as anodes in lithium-ion batteries, and they exhibit similar electrochemical behavior to metallic Li, indicating that the surface Li2CO3 layer is a good Li+ ion conductor. The air stability of the ASLSs is attributed to the surface Li2CO3 layer, which is barely soluble in water and does not react with oxygen and nitrogen in air at room temperature, thus passivating the Li core.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201807355-sup-0001-misc_information.pdf1.2 MB | Supplementary |
| anie201807355-sup-0001-Movie_S1.avi2.5 MB | Supplementary |
| anie201807355-sup-0001-Movie_S2.avi4.6 MB | Supplementary |
| anie201807355-sup-0001-Movie_S3.avi3.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. Xu, J. Wang, F. Ding, X. Chen, E. Nasybulin, Y. Zhang, J. G. Zhang, Energy Environ. Sci. 2014, 7, 513–537.
- 2
- 2aW.-S. Kim, W.-Y. Yoon, Electrochim. Acta 2004, 50, 541–545;
- 2bI. W. Seong, C. H. Hong, B. K. Kim, W. Y. Yoon, J. Power Sources 2008, 178, 769–773.
- 3T. Osaka, T. Momma, K. Nishimura, T. Tajima, J. Electrochem. Soc. 1993, 140, 2745–2748.
- 4K. Kanamura, S. Shiraishi, Z. Takehara, Cheminform 1994, 25, L108.
- 5M. Ishikawa, M. Morita, Y. Matsuda, J. Power Sources 1997, 68, 501–505.
- 6M. Morita, S. Aoki, Y. Matsuda, Electrochim. Acta 1992, 37, 119–123.
- 7C. Wang, H. Nakamura, H. Noguchi, M. Yoshio, Electrochemistry 1997, 65, 941–948.
- 8H. E. Park, I. W. Seong, W. Y. Yoon, J. Power Sources 2009, 189, 499–502.
- 9D.-R. Lru, D. B. Williams, Philos. Mag. B 1986, 53, L123–L128.
10.1080/13642818608240660 Google Scholar
- 10M. M. Markowitz, D. A. Boryta, J. Chem. Eng. Data 1962, 7, 586–591.
- 11W. Xu, N. Birbilis, G. Sha, Y. Wang, J. E. Daniels, Y. Xiao, M. Ferry, Nat. Mater. 2015, 14, 1229.
- 12D. W. Jeppson, J. L. Ballif, W. W. Yuan, B. E. Chou, Lithium literature review: lithium′s properties and interactions, No. HEDL-TME–78-15, Hanford Engineering Development Lab., 1978.
- 13
- 13aX. Wang, D.-M. Tang, H. Li, W. Yi, T. Zhai, Y. Bando, D. Golberg, Chem. Commun. 2012, 48, 4812–4814;
- 13bM. Sun, K. Qi, X. Li, Q. Huang, J. Wei, Z. Xu, W. Wang, X. Bai, ChemElectroChem 2016, 3, 1296–1300.
- 14
- 14aJ. Mizusaki, H. Tagawa, K. Saito, K. Uchida, M. Tezuka, Solid State Ionics 1992, s53-56, 791–797;
- 14bS. Shi, Y. Qi, H. Li, L. G. H. Jr, J. Phys. Chem. C 2013, 117, 8579–8593.
- 15S. W. Kim, Y. J. Ahn, W. Y. Yoon, Met. Mater. 2000, 6, 345–349.
- 16S. Bhattacharya, A. R. Riahi, A. T. Alpas, Carbon 2014, 77, 99–112.
- 17M. Asadi, B. Sayahpour, P. Abbasi, A. T. Ngo, K. Karis, J. R. Jokisaari, C. Liu, B. Narayanan, M. Gerard, P. Yasaei, X. Hu, A. Mukherjee, K. C. Lau, R. S. Assary, F. Khalili-Araghi, R. F. Klie, L. A. Curtiss, A. Salehi-Khojin, Nature 2018, 555, 502.
- 18
- 18aW. H. Woodford, W. C. Carter, Y. M. Chiang, Energy Environ. Sci. 2012, 5, 8014–8024;
- 18bW. H. Woodford, Y. M. Chiang, W. C. Carter, J. Electrochem. Soc. 2013, 160, A 1286–A1292.
- 19
- 19aV. Aravindan, Y. S. Lee, S. Madhavi, Adv. Energy Mater. 2017, 7, 1602607;
- 19bB. Xiang, L. Wang, G. Liu, A. M. Minor, J. Electrochem. Soc. 2013, 160, A 415–A419;
- 19cJ. T. Vaughey, G. Liu, J. G. Zhang, MRS Bull. 2014, 39, 429–435.