Release of Singlet Oxygen from Aromatic Endoperoxides by Chemical Triggers
Graphical Abstract
Abstract
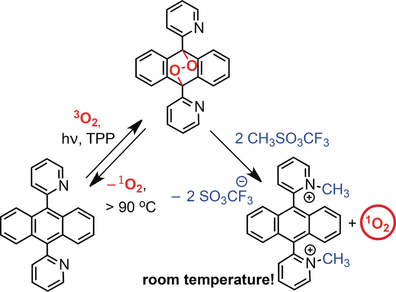
The generation of reactive singlet oxygen under mild conditions is of current interest in chemistry, biology, and medicine. We were able to release oxygen from dipyridylanthracene endoperoxides (EPOs) by using a simple chemical trigger at low temperature. Protonation and methylation of such EPOs strongly accelerated these reactions. Furthermore, the methyl pyridinium derivatives are water soluble and therefore serve as oxygen carriers in aqueous media. Methylation of the EPO of the ortho isomer affords the parent form directly without increasing the temperature under very mild conditions. This exceptional behavior is ascribed to the close contact between the nitrogen atom and the peroxo group. Singlet oxygen is released upon this reaction, and can be used to oxygenate an acceptor such as tetramethylethylene in the dark with no heating. Thus, a new chemical source of singlet oxygen has been found, which is triggered by a simple stimulus.