Docking Strategy To Construct Thermostable, Single-Crystalline, Hydrogen-Bonded Organic Framework with High Surface Area
Corresponding Author
Dr. Ichiro Hisaki
Department of Material and Life Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorYuto Suzuki
Department of Material and Life Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorEduardo Gomez
Departamento de Quimica Fisica, Facultad de Ciencias Ambientales y Bioquimica, and INAMOL, Universidad de Castilla-La Mancha, Avenida Carlos III, S/N, 45071 Toledo, Spain
Search for more papers by this authorDr. Boiko Cohen
Departamento de Quimica Fisica, Facultad de Ciencias Ambientales y Bioquimica, and INAMOL, Universidad de Castilla-La Mancha, Avenida Carlos III, S/N, 45071 Toledo, Spain
Search for more papers by this authorDr. Norimitsu Tohnai
Department of Material and Life Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Abderrazzak Douhal
Departamento de Quimica Fisica, Facultad de Ciencias Ambientales y Bioquimica, and INAMOL, Universidad de Castilla-La Mancha, Avenida Carlos III, S/N, 45071 Toledo, Spain
Search for more papers by this authorCorresponding Author
Dr. Ichiro Hisaki
Department of Material and Life Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorYuto Suzuki
Department of Material and Life Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorEduardo Gomez
Departamento de Quimica Fisica, Facultad de Ciencias Ambientales y Bioquimica, and INAMOL, Universidad de Castilla-La Mancha, Avenida Carlos III, S/N, 45071 Toledo, Spain
Search for more papers by this authorDr. Boiko Cohen
Departamento de Quimica Fisica, Facultad de Ciencias Ambientales y Bioquimica, and INAMOL, Universidad de Castilla-La Mancha, Avenida Carlos III, S/N, 45071 Toledo, Spain
Search for more papers by this authorDr. Norimitsu Tohnai
Department of Material and Life Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Abderrazzak Douhal
Departamento de Quimica Fisica, Facultad de Ciencias Ambientales y Bioquimica, and INAMOL, Universidad de Castilla-La Mancha, Avenida Carlos III, S/N, 45071 Toledo, Spain
Search for more papers by this authorGraphical Abstract
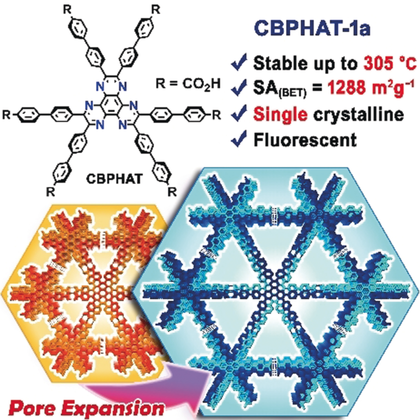
More than one HAT: Hexaazatriphenylene (HAT) derivatives are suitable building blocks for the systematic construction of stable hydrogen-bonded frameworks. The derivative with carboxybiphenyl groups forms a stable single-crystalline porous framework (CBPHAT-1 a) that displays protic solvent durability, heat resistance up to 305 °C, and SA(BET) of 1288 m2 g−1.
Abstract
Enhancing thermal and chemical durability and increasing surface area are two main directions for the construction and improvement of the performance of porous hydrogen-bonded organic frameworks (HOFs). Herein, a hexaazatriphenylene (HAT) derivative that possesses six carboxyaryl groups serves as a suitable building block for the systematic construction of thermally and chemically durable HOFs with high surface area through shape-fitted docking between the HAT cores and interpenetrated three-dimensional network. A HAT derivative with carboxybiphenyl groups forms a stable single-crystalline porous HOF that displays protic solvent durability, even in concentrated HCl, heat resistance up to 305 °C, and a high Brunauer–Emmett–Teller surface area [SA(BET)] of 1288 m2 g−1. A single crystal of this HOF displays anisotropic fluorescence, which suggests that it would be applicable to polarized emitters based on robust functional porous materials.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201805472-sup-0001-misc_information.pdf7.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. G. Slater, A. I. Cooper, Science 2015, 348, aaa 8075.
- 2
- 2aA. Comotti, A. Bracco, P. Sozzani, Acc. Chem. Res. 2016, 49, 1701–1710;
- 2bS. Das, P. Heasman, T. Ben, S. Qiu, Chem. Rev. 2017, 117, 1515–1563.
- 3
- 3aA. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger, O. M. Yaghi, Science 2005, 310, 1166–1170;
- 3bP. J. Waller, F. Gándara, O. M. Yaghi, Acc. Chem. Res. 2015, 48, 3053–3063.
- 4
- 4aX. Feng, X. Ding, D. Jiang, Chem. Soc. Rev. 2012, 41, 6010–6022;
- 4bS.-Y. Ding, W. Wang, Chem. Soc. Rev. 2013, 42, 548–568;
- 4cM. Dogru, T. Bein, Chem. Commun. 2014, 50, 5531–5546;
- 4dW. Zhao, L. Xia, X. Liu, CrystEngComm 2018, 20, 1613–1634.
- 5V. Nguyen, M. Grünwald, J. Am. Chem. Soc. 2018, 140, 3306–3311.
- 6Some excellent COF systems with high crystallinity have been reported. See, for example:
- 6aD. Beaudoin, T. Maris, J. D. Wuest, Nat. Chem. 2013, 5, 830–834;
- 6bP. Kissel, D. J. Murray, W. J. Wulftange, V. J. Catalano, B. T. King, Nat. Chem. 2014, 6, 774–778;
- 6cM. J. Kory, M. Wörle, T. Weber, P. Payamyar, S. W. van de Poll, J. Dshemuchadse, N. Trapp, A. D. Schlüter, Nat. Chem. 2014, 6, 779–784;
- 6dL. Ascherl, T. Sick, J. T. Margraf, S. H. Lapidus, M. Calik, C. Hettstedt, K. Karaghiosoff, M. Döblinger, T. Clark, K. W. Chapman, F. Auras, T. Bein, Nat. Chem. 2016, 8, 310–316.
- 7For a precise structural study based on theoretical calculations, see: B. Lukose, A. Kuc, T. Heine, Chem. Eur. J. 2011, 17, 2388–2392.
- 8Review of HOFs, see: J. Lu, R. Cao, Angew. Chem. Int. Ed. 2016, 55, 9474–9480; Angew. Chem. 2016, 128, 9624–9630.
- 9For supramolecular synthons, see: G. R. Desiraju, Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327; Angew. Chem. 1995, 107, 2541–2558.
- 10
- 10aA. R. A. Palmans, J. A. J. M. Vekemans, H. Kooijman, A. L. Spek, E. W. Meijer, Chem. Commun. 1997, 2247–2248;
- 10bP. Sozzani, A. Comotti, R. Simonutti, T. Meersmann, J. W. Logan, A. Pines, Angew. Chem. Int. Ed. 2000, 39, 2695–2699;
10.1002/1521-3773(20000804)39:15<2695::AID-ANIE2695>3.0.CO;2-M CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 2807–2810;
- 10cB. K. Saha, R. K. R. Jetti, K. S. Reddy, S. Aitipamula, A. Nangia, Cryst. Growth Des. 2005, 5, 887–899;
- 10dK. E. Maly, E. Gagnon, T. Maris, J. D. Wuest, J. Am. Chem. Soc. 2007, 129, 4306–4322;
- 10eJ. Yang, M. B. Dewal, S. Profeta, Jr., M. D. Smith, Y. Li, L. S. Shimizu, J. Am. Chem. Soc. 2008, 130, 612–621;
- 10fA. Comotti, S. Bracco, G. Distefano, P. Sozzani, Chem. Commun. 2009, 284–286;
- 10gW. Yang, A. Greenaway, X. Lin, R. Matsuda, A. J. Blake, C. Wilson, W. Lewis, P. Hubberstey, S. Kitagawa, N. R. Champness, M. Schröder, J. Am. Chem. Soc. 2010, 132, 14457–14469;
- 10hM. Mastalerz, I. Oppel, Angew. Chem. Int. Ed. 2012, 51, 5252–5255; Angew. Chem. 2012, 124, 5345–5348;
- 10iX.-Z. Luo, X.-J. Jia, J.-H. Deng, J.-L. Zhong, H.-J. Liu, K.-J. Wang, D.-C. Zhong, J. Am. Chem. Soc. 2013, 135, 11684–11687;
- 10jT.-H. Chen, I. Popov, W. Kaveevivitchai, Y.-C. Chuang, Y.-S. Chen, O. Daugulis, A. J. Jacobson, O. Š. Miljanić, Nat. Commun. 2014, 5, 5131;
- 10kJ. Lü, C. Perez-Krap, M. Suyetin, N. H. Alsmail, Y. Yan, S. Yang, W. Lewis, E. Bichoutskaia, C. C. Tang, A. J. Blake, R. Cao, M. Schröder, J. Am. Chem. Soc. 2014, 136, 12828–12831;
- 10lA. Comotti, S. Bracco, A. Yamamoto, M. Beretta, T. Hirukawa, N. Tohnai, M. Miyata, P. Sozzani, J. Am. Chem. Soc. 2014, 136, 618–621;
- 10mP. Li, Y. He, Y. Zhao, L. Weng, H. Wang, R. Krishna, H. Wu, W. Zhou, M. O'Keeffe, Y. Han, B. Chen, Angew. Chem. Int. Ed. 2015, 54, 574–577; Angew. Chem. 2015, 127, 584–587;
- 10nV. N. Yadav, A. Comotti, P. Sozzani, S. Bracco, T. Bonge-Hansen, M. Hennum, C. H. Görbitz, Angew. Chem. Int. Ed. 2015, 54, 15684–15688; Angew. Chem. 2015, 127, 15910–15914;
- 10oY. Zhou, B. Liu, X. Sun, J. Li, G. Li, Q. Huo, Y. Liu, Cryst. Growth Des. 2017, 17, 6653–6659;
- 10pS. Bracco, T. Miyano, M. Negroni, I. Bassanetti, L. Marchio’, P. Sozzani, N. Tohnai, A. Comotti, Chem. Commun. 2017, 53, 7776–7779;
- 10qM. I. Hashim, H. T. M. Le, T.-H. Chen, Y.-S. Chen, O. Daugulis, C.-W. Hsu, A. J. Jacobson, W. Kaveevivitchai, X. Liang, T. Makarenko, O. Š. Miljanić, I. Popovs, H. V. Tran, X. Wang, C.-H. Wu, J. I. Wu, J. Am. Chem. Soc. 2018, 140, 6014–6026.
- 11
- 11aY. He, S. Xiang, B. Chen, J. Am. Chem. Soc. 2011, 133, 14570–14573;
- 11bH. Wang, B. Li, H. Wu, T.-L. Hu, Z. Yao, W. Zhou, S. Xiang, B. Chen, J. Am. Chem. Soc. 2015, 137, 9963–9970;
- 11cW. Yang, B. Li, H. Wang, O. Alduhaish, K. Alfooty, M. A. Zayed, P. Li, H. D. Arman, B. Chen, Cryst. Growth Des. 2015, 15, 2000–2004.
- 12
- 12aD. J. Duchamp, R. Marsh, Acta Crystallogr. Sect. B 1969, 25, 5–19;
- 12bF. H. Herbstein, M. Kapon, G. M. Reisner, J. Inclusion Phenom. 1987, 5, 211–214;
- 12cK. Kobayashi, T. Shirasaka, E. Horn, N. Furukawa, Tetrahedron Lett. 2000, 41, 89–93;
- 12dC. A. Zentner, H. W. H. Lai, J. T. Greenfield, R. A. Wiscons, M. Zeller, C. F. Campana, O. Talu, S. A. FitzGerald, J. L. C. Rowsell, Chem. Commun. 2015, 51, 11642–11645;
- 12eS. Nandi, D. Chakraborty, R. Vaidhyanathan, Chem. Commun. 2016, 52, 7249–7252;
- 12fI. Hisaki, N. Q. E. Affendy, N. Tohnai, CrystEngComm 2017, 19, 4892–4898;
- 12gF. Hu, C. Liu, M. Wu, J. Pang, F. Jiang, D. Yuan, M. Hong, Angew. Chem. Int. Ed. 2017, 56, 2101–2104; Angew. Chem. 2017, 129, 2133–2136;
- 12hW. Yang, J. Wang, H. Wang, Z. Bao, J. C.-G. Zhao, B. Chen, Cryst. Growth Des. 2017, 17, 6132–6137.
- 13
- 13aI. Hisaki, S. Nakagawa, N. Tohnai, M. Miyata, Angew. Chem. Int. Ed. 2015, 54, 3008–3012; Angew. Chem. 2015, 127, 3051–3055;
- 13bI. Hisaki, N. Ikenaka, N. Tohnai, M. Miyata, Chem. Commun. 2016, 52, 300–303;
- 13cI. Hisaki, S. Nakagawa, N. Ikenaka, Y. Imamura, M. Katouda, M. Tashiro, H. Tsuchida, T. Ogoshi, H. Sato, N. Tohnai, M. Miyata, J. Am. Chem. Soc. 2016, 138, 6617–6628;
- 13dI. Hisaki, S. Nakagawa, H. Sato, N. Tohnai, Chem. Commun. 2016, 52, 9781–9784;
- 13eI. Hisaki, H. Toda, H. Sato, N. Tohnai, H. Sakurai, Angew. Chem. Int. Ed. 2017, 56, 15294–15298; Angew. Chem. 2017, 129, 15496–15500.
- 14I. Hisaki, N. Ikenaka, E. Gomez, B. Cohen, N. Tohnai, A. Douhal, Chem. Eur. J. 2017, 23, 11611–11619.
- 15D. Z. Rogers, J. Org. Chem. 1986, 51, 3904–3905.
- 16Crystal data for CBPHAT-1(TCB): (C90H54N6O12)⋅3 (C6H3Cl3), Fw=1955.70, a=b=29.7532(15) Å, c=7.1146(6) Å, α=β=90°, γ=120°, V=5454.4(6) Å3, T=93 K, trigonal, space group
 , Z=2, 29 812 collected, 7251 unique (Rint=0.097) reflections, the final R1 and wR2 values 0.130 (I>2.0σ(I)) and 0.385 (all data), respectively. Crystal data for CBPHAT-1 a: C90H54N6O12, Fw=1411.45, a=b=29.7810(10) Å, c=7.1709(3) Å, α=β=90°, γ=120°, V=5507.9(3) Å3, T=93 K, trigonal, space group
, Z=2, 29 812 collected, 7251 unique (Rint=0.097) reflections, the final R1 and wR2 values 0.130 (I>2.0σ(I)) and 0.385 (all data), respectively. Crystal data for CBPHAT-1 a: C90H54N6O12, Fw=1411.45, a=b=29.7810(10) Å, c=7.1709(3) Å, α=β=90°, γ=120°, V=5507.9(3) Å3, T=93 K, trigonal, space group  , Z=2, 16474 collected, 7205 unique (Rint=0.075) reflections, the final R1 and wR2 values 0.078 (I>2.0σ(I)) and 0.241 (all data). CCDC 1841012 and 1841011 ([CBPHAT-1(TCB)] and CBPHAT-1 a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
, Z=2, 16474 collected, 7205 unique (Rint=0.075) reflections, the final R1 and wR2 values 0.078 (I>2.0σ(I)) and 0.241 (all data). CCDC 1841012 and 1841011 ([CBPHAT-1(TCB)] and CBPHAT-1 a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 17A. L. Spek, Acta Crystallogr. Sect. D 2009, 65, 148–155.
- 18CBPHAT-1 a is hardly soluble in dichloromethane, while it is moderately soluble in DMF.
- 19Recently isostructural HOFs with large pores were reported, see Ref. [10q].