Single-Molecule Determination of the Isomers of d-Glucose and d-Fructose that Bind to Boronic Acids
Graphical Abstract
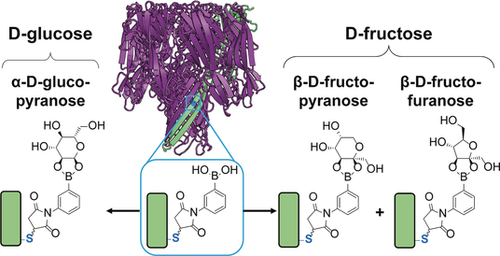
Sweet reaction: The aqueous, reversible covalent chemistry of boronic acids and diols can be monitored inside a protein nanoreactor. The approach is used to identify which cyclic isomers of d-glucose and d-fructose bind to a boronic acid in aqueous solution. Both of these binding modes contradict current binding models.
Abstract
Monosaccharides, such as d-glucose and d-fructose, exist in aqueous solution as an equilibrium mixture of cyclic isomers and can be detected with boronic acids by the reversible formation of boronate esters. The engineering of accurate, discriminating and continuous monitoring devices relies on knowledge of which cyclic isomer of a sugar binds to a boronic acid receptor. Herein, by monitoring fluctuations in ionic current, we show that an engineered α-hemolysin (αHL) nanopore modified with a boronic acid reacts reversibly with d-glucose as the pyranose isomer (α-d-glucopyranose) and d-fructose as either the furanose (β-d-fructofuranose) or the pyranose (β-d-fructopyranose). Both of these binding modes contradict current binding models. With this knowledge, we distinguished the individual sugars in a mixture of d-maltose, d-glucose, and d-fructose.