Combining Organocatalysis and Lanthanide Catalysis: A Sequential One-Pot Quadruple Reaction Sequence/Hetero-Diels–Alder Asymmetric Synthesis of Functionalized Tricycles
Dr. Simon Dochain
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorM. Sc. Fabrizio Vetica
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorDr. Rakesh Puttreddy
Department of Chemistry, Nanoscience Center, University of Jyvaskyla, 40014 JYU Finland
Search for more papers by this authorProf. Dr. Kari Rissanen
Department of Chemistry, Nanoscience Center, University of Jyvaskyla, 40014 JYU Finland
Search for more papers by this authorCorresponding Author
Prof. Dr. Dieter Enders
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorDr. Simon Dochain
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorM. Sc. Fabrizio Vetica
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorDr. Rakesh Puttreddy
Department of Chemistry, Nanoscience Center, University of Jyvaskyla, 40014 JYU Finland
Search for more papers by this authorProf. Dr. Kari Rissanen
Department of Chemistry, Nanoscience Center, University of Jyvaskyla, 40014 JYU Finland
Search for more papers by this authorCorresponding Author
Prof. Dr. Dieter Enders
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorGraphical Abstract
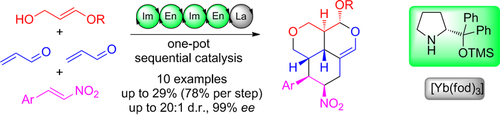
Targeting complexity: A unique combination of organocatalysis with lanthanide catalysis directly leads to complex tricyclic structures from readily available and simple compounds. Six new bonds and six stereocenters are formed with virtually complete stereoselectivity in this multicomponent one-pot procedure. fod=2,2-dimethyl-6,6,7,7,8,8,8-heptafluorooctane-3,5-dionato.
Abstract
A stereoselective one-pot synthesis of functionalized complex tricyclic polyethers has been achieved using the combination of secondary amine and lanthanide catalysis. This one-pot quadruple reaction/Hetero-Diels–Alder sequence gave good yields (per step) as well as excellent diastereo- and enantioselectivities. Furthermore, the particular combination of lanthanide complexes with organocatalysis is one of the first examples described for sequential catalysis.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201610196-sup-0001-misc_information.pdf3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. M. P. Koskinen in Asymmetric Synthesis of Natural Products, 2nd ed., Wiley-VCH, Weinheim, 2012.
10.1002/9781118347300 Google Scholar
- 2
- 2aD. Enders, C. Grondal, M. R. M. Hüttl, Angew. Chem. Int. Ed. 2007, 46, 1570; Angew. Chem. 2007, 119, 1590;
- 2bŁ. Albrecht, H. Jiang, K. A. Jørgensen, Angew. Chem. Int. Ed. 2011, 50, 8492; Angew. Chem. 2011, 123, 8642;
- 2cP.-F. Xu, W. Wang in Catalytic Cascade Reactions, Wiley-VCH, Weinheim, 2014;
- 2dC. M. R. Volla, I. Atodiresei, M. Rueping, Chem. Rev. 2014, 114, 2390;
- 2eT. Broja, P. J. W. Fuchs, K. Zeitler, Nat. Chem. 2015, 7, 950.
- 3For selected books on organocatalysis, see:
- 3a Asymmetric Organocatalysis (Eds.: ), Wiley-VCH, Weinheim, 2005;
- 3b Organocatalysis (Eds.: ), Springer, Berlin, 2007;
- 3c Asymmetric Organocatalysis (Ed.: ), Springer, Berlin, 2010;
- 3d Enantioselective Organocatalyzed Reactions II (Ed.: ), Springer, Berlin, 2011;
- 3e Comprehensive Enantioselective Organocatalysis, Vol. 3 (Ed.: ), Wiley-VCH, Weinheim, 2013;
- 3f Stereoselective Organocatalysis (Ed.: ), Wiley, Hoboken, 2013.
- 4For recent reviews on the combination of transition-metal catalysis with organoatalysis, see:
- 4aC. Zhong, X. Shi, Eur. J. Org. Chem. 2010, 2999;
- 4bC. C. J. Loh, D. Enders, Chem. Eur. J. 2012, 18, 10212;
- 4cZ. Du, Z. Shao, Chem. Soc. Rev. 2013, 42, 1337;
- 4dA. Córdova, Pure Appl. Chem. 2015, 87, 1011.
- 5Y. Hayashi, Chem. Sci. 2016, 7, 866.
- 6
- 6aJ. M. Lee, Y. Na, H. Han, S. Chang, Chem. Soc. Rev. 2004, 33, 302;
- 6bZ. Shao, H. Zhang, Chem. Soc. Rev. 2009, 38, 2745.
- 7For selected domino reactions, see:
- 7aD. Enders, M. R. M. Hüttl, C. Grondal, G. Raabe, Nature 2006, 441, 861;
- 7bD. Enders, M. R. M. Hüttl, G. Raabe, J. W. Bats, Adv. Synth. Catal. 2008, 350, 267;
- 7cD. Enders, C. Wang, M. Mukanova, A. Greb, Chem. Commun. 2010, 46, 2447;
- 7dX. Zeng, Q. Ni, G. Raabe, D. Enders, Angew. Chem. Int. Ed. 2013, 52, 2977; Angew. Chem. 2013, 125, 3050.
- 8For sequential catalysis, see:
- 8aC. C. J. Loh, J. Badorrek, G. Raabe, D. Enders, Chem. Eur. J. 2011, 17, 13409;
- 8bD. Hack, C. C. J. Loh, J. M. Hartmann, G. Raabe, D. Enders, Chem. Eur. J. 2014, 20, 3917;
- 8cD. Hack, P. Chauhan, K. Deckers, G. N. Hermann, L. Mertens, G. Raabe, D. Enders, Org. Lett. 2014, 16, 5188;
- 8dD. Hack, P. Chauhan, K. Deckers, Y. Mizutani, G. Raabe, D. Enders, Chem. Commun. 2015, 51, 2266;
- 8eU. Kaya, P. Chauhan, D. Hack, K. Deckers, R. Puttreddy, K. Rissanen, D. Enders, Chem. Commun. 2016, 52, 1669;
- 8fD. Hack, A. B. Dürr, K. Deckers, P. Chauhan, N. Seling, L. Rübenach, L. Mertens, G. Raabe, F. Schoenebeck, D. Enders, Angew. Chem. Int. Ed. 2016, 55, 1797; Angew. Chem. 2016, 128, 1829.
- 9
- 9aC.-H. Huo, D. Guo, L.-R. Shen, B.-W. Yin, F. Sauriol, L.-G. Li, M.-L. Zhang, Q.-W. Shi, H. Kiyota, Tetrahedron Lett. 2010, 51, 754;
- 9bA. Roy, S. Saraf, Biol. Pharm. Bull. 2006, 29, 191.
- 10F.-L. Zhang, A.-W. Xu, Y.-F. Gong, M.-H. Wei, X.-L. Yang, Chem. Eur. J. 2009, 15, 6815.
- 11For selected reviews on asymmetric catalyzed Diels–Alder and IEDHDA reactions, see:
- 11aH. B. Kagan, O. Riant, Chem. Rev. 1992, 92, 1007;
- 11bX. Jiang, R. Wang, Chem. Rev. 2013, 113, 5515;
- 11cH. Du, K. Ding in Handbook of Cyclization Reactions, Vol. 1 (Ed.: ), Wiley-VCH, Weinheim, 2010, pp. 1–57.
- 12For lanthanide-catalyzed Diels–Alder reactions, see:
- 12aS. Kobayashi, I. Hachiya, T. Takahori, M. Araki, H. Ishitani, Tetrahedron Lett. 1992, 33, 6815;
- 12bS. Kobayashi, I. Hachiya, H. Ishitani, M. Araki, Tetrahedron Lett. 1993, 34, 4535;
- 12cD. A. Powell, R. A. Batey, Tetrahedron Lett. 2003, 44, 7569;
- 12dG. Desimoni, G. Faita, M. Mella, F. Piccinini, M. Toscanini, Eur. J. Org. Chem. 2007, 1529.
- 13For lanthanide-catalyzed IEDHDA reactions, see:
- 13aS. Danishefsky, M. Bednarski, Tetrahedron Lett. 1984, 25, 721;
- 13bI. E. Markó, G. R. Evans, Tetrahedron Lett. 1994, 35, 2767;
- 13cI. E. Markó, G. R. Evans, Tetrahedron Lett. 1994, 35, 2771;
- 13dC. Spino, L. Clouston, D. Berg, Can. J. Chem. 1997, 75, 1047.
- 14During our investigation on the combination of the two protocols, the Jørgensen group published a similar approach for the construction of tetrahydroisochromenes: N. Hammer, L. A. Leth, J. Stiller, M. E. Jensen, K. A. Jørgensen, Chem. Sci. 2016, 7, 3649.
- 15See the Supporting Information.
- 16CCDC 1510190 (6 a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.