Tailoring Renal Clearance and Tumor Targeting of Ultrasmall Metal Nanoparticles with Particle Density
Dr. Shaoheng Tang
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorChuanqi Peng
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorJing Xu
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorBujie Du
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorQingxiao Wang
Department of Materials Science and Engineering, The University of Texas at Dallas, USA
Search for more papers by this authorRodrigo D. Vinluan III
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorDr. Mengxiao Yu
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorProf. Dr. Moon J. Kim
Department of Materials Science and Engineering, The University of Texas at Dallas, USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jie Zheng
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorDr. Shaoheng Tang
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorChuanqi Peng
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorJing Xu
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorBujie Du
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorQingxiao Wang
Department of Materials Science and Engineering, The University of Texas at Dallas, USA
Search for more papers by this authorRodrigo D. Vinluan III
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorDr. Mengxiao Yu
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorProf. Dr. Moon J. Kim
Department of Materials Science and Engineering, The University of Texas at Dallas, USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jie Zheng
Department of Chemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX, 75080 USA
Search for more papers by this authorGraphical Abstract
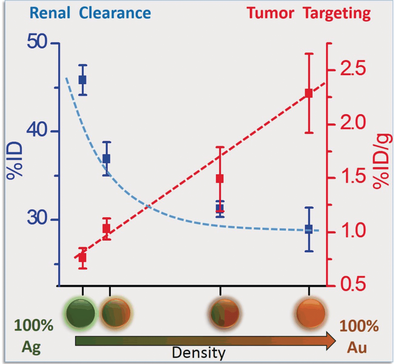
Density matters: Four different metal nanoparticles with the same size and surface chemistry but different densities were synthesized. It was then shown that the renal clearance of ultrasmall metal nanoparticles decreases exponentially with an increase in particle density while tumor targeting linearly increases.
Abstract
Identifying key factors that govern the in vivo behavior of nanomaterials is critical to the clinical translation of nanomedicines. Overshadowed by size-, shape-, and surface-chemistry effects, the impact of the particle core density on clearance and tumor targeting of inorganic nanoparticles (NPs) remains largely unknown. By utilizing a class of ultrasmall metal NPs with the same size and surface chemistry but different densities, we found that the renal-clearance efficiency exponentially increased in the early elimination phase while passive tumor targeting linearly decreased with a decrease in particle density. Moreover, lower-density NPs are more easily distributed in the body and have shorter retention times in highly permeable organs than higher-density NPs. The density-dependent in vivo behavior of metal NPs likely results from their distinct margination in laminar blood flow, which opens up a new path for precise control of nanomedicines in vivo.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201609043-sup-0001-misc_information.pdf4.8 MB | Supplementary |
| anie201609043-sup-0001-Movie_S1.avi10.6 MB | Supplementary |
| anie201609043-sup-0001-Movie_S2.avi11 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak, W. C. W. Chan, Nat. Rev. Mater. 2016, 5, 16014–16026.
- 2
- 2aY. N. Zhang, W. Poon, A. J. Tavares, I. D. McGilvray, W. C. W. Chan, J. Controlled Release 2016, 1, 1–17;
- 2bB. D. Chithrani, A. A. Ghazani, W. C. W. Chan, Nano Lett. 2006, 6, 662–668;
- 2cL. Y. T. Chou, W. C. W. Chan, Adv. Healthcare Mater. 2012, 1, 714–721;
- 2dB. H. Kim, M. J. Hackett, J. Park, T. Hyeon, Chem. Mater. 2014, 26, 59–71;
- 2eE. A. Sykes, J. Chen, G. Zheng, W. C. W. Chan, ACS Nano 2014, 8, 5696–5706;
- 2fJ. Lazarovits, Y. Y. Chen, E. A. Sykes, W. C. W. Chan, Chem. Commun. 2015, 51, 2756–2767;
- 2gE. Huynh, M. A. Rajora, G. Zheng, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 6, 796–813.
- 3S. D. Perrault, C. Walkey, T. Jennings, H. C. Fischer, W. C. W. Chan, Nano Lett. 2009, 9, 1909–1915.
- 4K. C. L. Black, Y. Wang, H. P. Luehmann, X. Cai, W. Xing, B. Pang, Y. Zhao, C. S. Cutler, L. V. Wang, Y. Liu, Y. Xia, ACS Nano 2014, 8, 4385–4394.
- 5
- 5aR. Mo, T. Jiang, Z. Gu, Angew. Chem. Int. Ed. 2014, 53, 5815–5820; Angew. Chem. 2014, 126, 5925–5930;
- 5bY. Lu, Q. Hu, Y. Lin, D. B. Pacardo, C. Wang, W. Sun, F. S. Ligler, M. D. Dickey, Z. Gu, Nat. Commun. 2015, 6, 10066;
- 5cW. Sun, W. Ji, J. M. Hall, Q. Hu, C. Wang, C. L. Beisel, Z. Gu, Angew. Chem. Int. Ed. 2015, 54, 12029–12033; Angew. Chem. 2015, 127, 12197–12201;
- 5dJ. Yu, Y. Zhang, Y. Ye, R. DiSanto, W. Sun, D. Ranson, F. S. Ligler, J. B. Buse, Z. Gu, Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265.
- 6
- 6aP. Decuzzi, S. Lee, B. Bhushan, M. Ferrari, Ann. Biomed. Eng. 2005, 33, 179–190;
- 6bR. Toy, E. Hayden, C. Shoup, H. Baskaran, E. Karathanasis, Nanotechnology 2011, 22, 115101;
- 6cA. J. Thompson, O. Eniola-Adefeso, Acta Biomater. 2015, 21, 99–108.
- 7
- 7aH. S. Choi, B. I. Ipe, P. Misra, J. H. Lee, M. G. Bawendi, J. V. Frangioni, Nano Lett. 2009, 9, 2354–2359;
- 7bX. Huang, F. Zhang, L. Zhu, K. Y. Choi, N. Guo, J. Guo, K. Tackett, P. Anilkumar, G. Liu, Q. Quan, H. S. Choi, G. Niu, Y.-P. Sun, S. Lee, X. Chen, ACS Nano 2013, 7, 5684–5693;
- 7cH. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. I. Ipe, M. G. Bawendi, J. V. Frangioni, Nat. Biotechnol. 2007, 25, 1165–1170;
- 7dY. Yang, J. Liu, C. Liang, L. Feng, T. Fu, Z. Dong, Y. Chao, Y. Li, G. Lu, M. Chen, Z. Liu, ACS Nano 2016, 10, 2774–2781;
- 7eM. Zhou, J. Li, S. Liang, A. K. Sood, D. Liang, C. Li, ACS Nano 2015, 9, 7085–7096.
- 8H. S. Choi, W. Liu, F. Liu, K. Nasr, P. Misra, M. G. Bawendi, J. V. Frangioni, Nat. Nanotechnol. 2010, 5, 42–47.
- 9A. A. Burns, J. Vider, H. Ow, E. Herz, O. Penate-Medina, M. Baumgart, S. M. Larson, U. Wiesner, M. Bradbury, Nano Lett. 2009, 9, 442–448.
- 10
- 10aJ. Liu, M. Yu, X. Ning, C. Zhou, S. Yang, J. Zheng, Angew. Chem. Int. Ed. 2013, 52, 12572–12576; Angew. Chem. 2013, 125, 12804–12808;
- 10bC. Zhou, M. Long, Y. Qin, X. Sun, J. Zheng, Angew. Chem. Int. Ed. 2011, 50, 3168–3172; Angew. Chem. 2011, 123, 3226–3230;
- 10cM. Yu, J. Liu, X. Ning, J. Zheng, Angew. Chem. Int. Ed. 2015, 54, 15434–15438; Angew. Chem. 2015, 127, 15654–15658;
- 10dJ. Liu, P. N. Duchesne, M. Yu, X. Jiang, X. Ning, R. D. Vinluan, P. Zhang, J. Zheng, Angew. Chem. Int. Ed. 2016, 55, 8894–8898; Angew. Chem. 2016, 128, 9040–9044;
- 10eS. Sun, X. Ning, G. Zhang, Y.-C. Wang, C. Peng, J. Zheng, Angew. Chem. Int. Ed. 2016, 55, 2421–2424; Angew. Chem. 2016, 128, 2467–2470;
- 10fM. Yu, J. Zhou, B. Du, X. Ning, C. Authement, L. Gandee, P. Kapur, J.-T. Hsieh, J. Zheng, Angew. Chem. Int. Ed. 2016, 55, 2787–2791; Angew. Chem. 2016, 128, 2837–2841.
- 11
- 11aH. Chen, G. D. Wang, W. Tang, T. Todd, Z. Zhen, C. Tsang, K. Hekmatyar, T. Cowger, R. B. Hubbard, W. Zhang, J. Stickney, B. Shen, J. Xie, Adv. Mater. 2014, 26, 6761–6766;
- 11bS. Tang, M. Chen, N. Zheng, Small 2014, 10, 3139–3144.
- 12C. Zhou, G. Hao, P. Thomas, J. Liu, M. Yu, S. Sun, O. K. Öz, X. Sun, J. Zheng, Angew. Chem. Int. Ed. 2012, 51, 10118–10122; Angew. Chem. 2012, 124, 10265–10269.
- 13S. Yang, S. Sun, C. Zhou, G. Hao, J. Liu, S. Ramezani, M. Yu, X. Sun, J. Zheng, Bioconjugate Chem. 2015, 26, 511–519.
- 14D. J. Greenblatt, Annu. Rev. Med. 1985, 36, 421–427.
- 15S.-D. Li, L. Huang, Mol. Pharmaceutics 2008, 5, 496–504.