The Structural Basis of Transcription: 10 Years After the Nobel Prize in Chemistry
Merle Hantsche
Abteilung für Molekularbiologie, Max Planck Institut für biophysikalische Chemie, Am Fassberg 11, 37077 Göttingen, Germany
Search for more papers by this authorCorresponding Author
Prof. Patrick Cramer
Abteilung für Molekularbiologie, Max Planck Institut für biophysikalische Chemie, Am Fassberg 11, 37077 Göttingen, Germany
Search for more papers by this authorMerle Hantsche
Abteilung für Molekularbiologie, Max Planck Institut für biophysikalische Chemie, Am Fassberg 11, 37077 Göttingen, Germany
Search for more papers by this authorCorresponding Author
Prof. Patrick Cramer
Abteilung für Molekularbiologie, Max Planck Institut für biophysikalische Chemie, Am Fassberg 11, 37077 Göttingen, Germany
Search for more papers by this authorGraphical Abstract
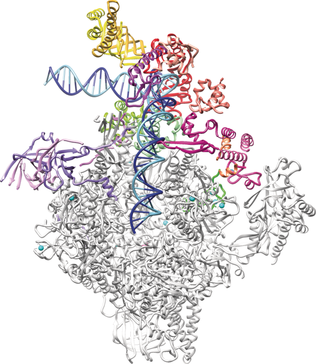
Transcription unlimited: In the 10 years since the Nobel Prize in Chemistry was awarded to Roger Kornberg for his studies of the molecular basis of transcription, significant advances have been made in our understanding of many aspects of transcription, and progess in cryo-electron microscopy has enabled the study of large molecular assemblies at high resolution. Our current understanding of the structural basis of transcription is discussed in this Minireview.
Abstract
Transcription is the first step in the expression of genetic information in all living cells. The regulation of transcription underlies cell differentiation, organism development, and the responses of living systems to changes in the environment. During transcription, the enzyme RNA polymerase uses DNA as a template to synthesize a complementary RNA copy from a gene. Herein, we summarize the progress in our understanding of the structural basis of eukaryotic gene transcription that has been made in the ten years since the Nobel Prize in Chemistry was given to Roger Kornberg in 2006. The basis for transcription initiation and RNA chain elongation is emerging, but the intricate mechanisms of transcription regulation remain to be elucidated. The field has also developed hybrid methods for structural biology that combine several techniques to determine the three-dimensional architecture of large and transient macromolecular assemblies.
References
- 1
- 1aR. D. Kornberg, Proc. Natl. Acad. Sci. USA 2007, 104, 12955–12961;
- 1bR. D. Kornberg, Angew. Chem. Int. Ed. 2007, 46, 6956–6065; Angew. Chem. 2007, 119, 7082–7092;
- 1chttp://nobelfoundation.org.
- 2
- 2aP. Cramer, D. A. Bushnell, J. Fu, A. L. Gnatt, B. Maier-Davis, N. E. Thompson, R. R. Burgess, A. M. Edwards, P. R. David, R. D. Kornberg, Science 2000, 288, 640–649;
- 2bP. Cramer, D. A. Bushnell, R. D. Kornberg, Science 2001, 292, 1863–1876;
- 2cA. L. Gnatt, P. Cramer, J. Fu, D. A. Bushnell, R. D. Kornberg, Science 2001, 292, 1876.
- 3G. Zhang, E. A. Campbell, L. Minakhin, C. Richter, K. Severinov, S. A. Darst, Cell 1999, 98, 811–824.
- 4
- 4aW. Kühlbrandt, eLife 2014, 3, e 03678;
- 4bE. Nogales, S. H. W. Scheres, Mol. Cell 2015, 58, 677–689.
- 5
- 5aR. G. Roeder, Trends Biochem. Sci. 1996, 21, 327–335;
- 5bS. Grünberg, S. Hahn, Trends Biochem. Sci. 2013, 38, 603–611;
- 5cS. Sainsbury, C. Bernecky, P. Cramer, Nat. Rev. Mol. Cell Biol. 2015, 16, 129–143.
- 6S. Buratowski, S. Hahn, L. Guarente, P. A. Sharp, Cell 1989, 56, 549–561.
- 7R. C. Conaway, J. W. Conaway, Annu. Rev. Biochem. 1993, 62, 161–190.
- 8
- 8aK. J. Armache, H. Kettenberger, P. Cramer, Proc. Natl. Acad. Sci. USA 2003, 100, 6964–6968;
- 8bD. A. Bushnell, R. D. Kornberg, Proc. Natl. Acad. Sci. USA 2003, 100, 6969–6973.
- 9
- 9aS. T. Smale, J. T. Kadonaga, Annu. Rev. Biochem. 2003, 72, 449–479;
- 9bJ. T. Kadonaga, Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 40–51.
- 10A. D. Basehoar, S. J. Zanton, B. F. Pugh, Cell 2004, 116, 699–709.
- 11
- 11aJ. L. Kim, D. B. Nikolov, S. K. Burley, Nature 1993, 365, 520–527;
- 11bY. Kim, J. H. Geiger, S. Hahn, P. B. Sigler, Nature 1993, 365, 512–520;
- 11cJ. L. Kim, S. K. Burley, Nat. Struct. Biol. 1994, 1, 638–653;
- 11dD. B. Nikolov, H. Chen, E. D. Halay, A. Hoffman, R. G. Roeder, S. K. Burley, Proc. Natl. Acad. Sci. USA 1996, 93, 4862–4867;
- 11eZ. S. Juo, T. K. Chiu, P. M. Leiberman, I. Baikalov, A. J. Berk, R. E. Dickerson, J. Mol. Biol. 1996, 261, 239–254;
- 11fG. A. Patikoglou, J. L. Kim, L. Sun, S. H. Yang, T. Kodadek, S. K. Burley, Genes Dev. 1999, 13, 3217–3230.
- 12
- 12aS. Tan, Y. Hunziker, D. F. Sargent, T. J. Richmond, Nature 1996, 381, 127–134;
- 12bJ. H. Geiger, S. Hahn, S. Lee, P. B. Sigler, Science 1996, 272, 830–836;
- 12cM. Bleichenbacher, S. Tan, T. J. Richmond, J. Mol. Biol. 2003, 332, 783–793.
- 13
- 13aO. Littlefield, Y. Korkhin, P. B. Sigler, Proc. Natl. Acad. Sci. USA 1999, 96, 13668–13673;
- 13bF. T. Tsai, P. B. Sigler, EMBO J. 2000, 19, 25–36;
- 13cT. Lagrange, A. N. Kapanidis, H. Tang, D. Reinberg, R. H. Ebright, Genes Dev. 1998, 12, 34–44;
- 13dW. Deng, S. G. E. Roberts, Genes Dev. 2005, 19, 2418–2423.
- 14H. S. Rhee, B. F. Pugh, Nature 2012, 483, 295–301.
- 15L. Tora, Genes Dev. 2002, 16, 673–675.
- 16
- 16aG. E. Chalkley, C. P. Verrijzer, EMBO J. 1999, 18, 4835–4845;
- 16bJ. W. M. Theisen, C. Y. Lim, J. T. Kadonaga, Mol. Cell. Biol. 2010, 30, 3471–3479;
- 16cT. W. Burke, J. T. Kadonaga, Genes Dev. 1997, 11, 3020–3031;
- 16dD.-h. Lee, N. Gershenzon, M. Gupta, P. Ilya, D. Reinberg, B. A. Lewis, I. P. Ioshikhes, Mol. Cell. Biol. 2005, 25, 9674–9686.
- 17
- 17aF. Andel III, A. G. Ladurner, C. Inouye, R. Tjian, E. Nogales, Science 1999, 286, 2153–2156;
- 17bP. Grob, M. J. Cruse, C. Inouye, M. Peris, P. A. Penczek, R. Tjian, E. Nogales, Structure 2006, 14, 511–520;
- 17cH. Elmlund, V. Baraznenok, T. Linder, Z. Szilagyi, R. Rofougaran, A. Hofer, H. Hebert, M. Lindahl, C. M. Gustafsson, Structure 2009, 17, 1442–1452;
- 17dC. Bieniossek, G. Papai, C. Schaffitzel, F. Garzoni, M. Chaillet, E. Scheer, P. Papadopoulos, L. Tora, P. Schultz, I. Berger, Nature 2013, 493, 699–702;
- 17eM. A. Cianfrocco, G. A. Kassavetis, P. Grob, J. Fang, T. Juven-Gershon, J. T. Kadonaga, E. Nogales, Cell 2013, 152, 120–131.
- 18R. K. Louder, Y. He, J. R. López-Blanco, J. Fang, P. Chacón, E. Nogales, Nature 2016, 1–16.
- 19
- 19aH. T. Chen, S. Hahn, Mol. Cell 2003, 12, 437–447;
- 19bD. A. Bushnell, K. D. Westover, R. E. Davis, R. D. Kornberg, Science 2004, 303, 983–988.
- 20
- 20aH. T. Chen, S. Hahn, Cell 2004, 119, 169–180;
- 20bG. Miller, S. Hahn, Nat. Struct. Mol. Biol. 2006, 13, 603–610.
- 21
- 21aD. Kostrewa, M. E. Zeller, K.-J. Armache, M. Seizl, K. Leike, M. Thomm, P. Cramer, Nature 2009, 462, 323–330;
- 21bX. Liu, D. A. Bushnell, D. Wang, G. Calero, R. D. Kornberg, Science 2010, 327, 206–209.
- 22C. Giardina, J. T. Lis, Science 1993, 261, 759–762.
- 23S. Sainsbury, J. Niesser, P. Cramer, Nature 2013, 493, 437–440.
- 24
- 24aT. Kim, R. H. Ebright, D. Reinberg, Science 2000, 288, 1418–1421;
- 24bH.-T. Chen, L. Warfield, S. Hahn, Nat. Struct. Mol. Biol. 2007, 14, 696–703;
- 24cZ. A. Chen, A. Jawhari, L. Fischer, C. Buchen, S. Tahir, T. Kamenski, M. Rasmussen, L. Lariviere, J.-C. Bukowski-Wills, M. Nilges, P. Cramer, J. Rappsilber, EMBO J. 2010, 29, 717–726;
- 24dJ. Eichner, H.-T. Chen, L. Warfield, S. Hahn, EMBO J. 2010, 29, 706–716;
- 24eS. Grünberg, L. Warfield, S. Hahn, Nat. Struct. Mol. Biol. 2012, 19, 788–796;
- 24fJ. Fishburn, S. Hahn, Mol. Cell. Biol. 2012, 32, 12–25;
- 24gW. Mühlbacher, S. Sainsbury, M. Hemann, M. Hantsche, S. Neyer, F. Herzog, P. Cramer, Nat. Commun. 2014, 5, 4310.
- 25Y. He, J. Fang, D. J. Taatjes, E. Nogales, Nature 2013, 495, 481–486.
- 26
- 26aC. Bernecky, F. Herzog, W. Baumeister, J. M. Plitzko, P. Cramer, Nature 2016, 529, 551–554;
- 26bK. Murakami, K.-L. Tsai, N. Kalisman, D. A. Bushnell, F. J. Asturias, R. D. Kornberg, Proc. Natl. Acad. Sci. USA 2015, 112, 13543–13548;
- 26cC. Plaschka, L. Larivière, L. Wenzeck, M. Seizl, M. Hemann, D. Tegunov, E. V. Petrotchenko, C. H. Borchers, W. Baumeister, F. Herzog, E. Villa, P. Cramer, Nature 2015, 518, 376–380.
- 27
- 27aL. Fan, A. S. Arvai, P. K. Cooper, S. Iwai, F. Hanaoka, J. A. Tainer, Mol. Cell 2006, 22, 27–37;
- 27bF. Gaiser, S. Tan, T. J. Richmond, J. Mol. Biol. 2000, 302, 1119–1127;
- 27cC. M. Groft, S. N. Uljon, R. Wang, M. H. Werner, Proc. Natl. Acad. Sci. USA 1998, 95, 9117–9122;
- 27dK. Kamada, J. De Angelis, R. G. Roeder, S. K. Burley, Proc. Natl. Acad. Sci. USA 2001, 98, 3115–3120;
- 27eA. M. Kilpatrick, L. M. I. Koharudin, G. A. Calero, A. M. Gronenborn, Proteins 2012, 80, 519–529;
- 27fA. Meinhart, J. Blobel, P. Cramer, J. Biol. Chem. 2003, 278, 48267–48274;
- 27gM. Okuda, A. Tanaka, Y. Arai, M. Satoh, H. Okamura, A. Nagadoi, F. Hanaoka, Y. Ohkuma, Y. Nishimura, J. Biol. Chem. 2004, 279, 51395–51403;
- 27hM. Okuda, Y. Watanabe, H. Okamura, F. Hanaoka, Y. Ohkuma, Y. Nishimura, EMBO J. 2000, 19, 1346–1356.
- 28C. Plaschka, M. Hantsche, C. Dienemann, C. Burzinski, J. Plitzko, P. Cramer, Nature 2016, 1, 1–22.
- 29Y. He, C. Yan, J. Fang, C. Inouye, R. Tjian, I. Ivanov, E. Nogales, Nature 2016, 533, 359–365.
- 30
- 30aM. E. Maxon, J. A. Goodrich, R. Tjian, Genes Dev. 1994, 8, 515–524;
- 30bM. Okuda, A. Tanaka, M. Satoh, S. Mizuta, M. Takazawa, Y. Ohkuma, Y. Nishimura, EMBO J. 2008, 27, 1161–1171.
- 31J. M. Egly, F. Coin, DNA Repair 2011, 10, 714–721.
- 32J. Fishburn, E. Tomko, E. Galburt, S. Hahn, Proc. Natl. Acad. Sci. USA 2015, 112, 3961–3966.
- 33
- 33aS. Buratowski, M. Sopta, J. Greenblatt, P. A. Sharp, Proc. Natl. Acad. Sci. USA 1991, 88, 7509–7513;
- 33bF. C. Holstege, D. Tantin, M. Carey, D. van V, H. T. Timmers, EMBO J. 1995, 14, 810–819.
- 34
- 34aR. D. Kornberg, Trends Biochem. Sci. 2005, 30, 235–239;
- 34bS. Malik, R. G. Roeder, Nat. Rev. Genet. 2010, 11, 761–772;
- 34cR. C. Conaway, J. W. Conaway, Semin. Cell Dev. Biol. 2011, 22, 729–734;
- 34dB. L. Allen, D. J. Taatjes, Nat. Rev. Mol. Cell Biol. 2015, 16, 155–166.
- 35
- 35aP. M. Flanagan, R. J. Kelleher, M. H. Sayre, H. Tschochner, R. D. Kornberg, Nature 1991, 354, 436–438;
- 35bY. J. Kim, S. Björklund, Y. Li, M. H. Sayre, R. D. Kornberg, Cell 1994, 77, 599–608.
- 36F. J. Asturias, Y. W. Jiang, L. C. Myers, C. M. Gustafsson, R. D. Kornberg, Science 1999, 283, 985–987.
- 37
- 37aC. Bernecky, P. Grob, C. C. Ebmeier, E. Nogales, D. J. Taatjes, PLoS Biol. 2011, 9, e 1000603;
- 37bG. Cai, Y. L. Chaban, T. Imasaki, J. A. Kovacs, G. Calero, P. A. Penczek, Y. Takagi, F. J. Asturias, Structure 2012, 20, 899–910;
- 37cG. Cai, T. Imasaki, Y. Takagi, F. J. Asturias, Structure 2009, 17, 559–567;
- 37dG. Cai, T. Imasaki, K. Yamada, F. Cardelli, Y. Takagi, F. J. Asturias, Nat. Struct. Mol. Biol. 2010, 17, 273–279;
- 37eJ. A. Davis, Y. Takagi, R. D. Kornberg, F. J. Asturias, Mol. Cell 2002, 10, 409–415;
- 37fH. Elmlund, V. Baraznenok, M. Lindahl, C. O. Samuelsen, P. J. B. Koeck, S. Holmberg, H. Hebert, C. M. Gustafsson, Proc. Natl. Acad. Sci. USA 2006, 103, 15788–15793;
- 37gK. L. Tsai, C. Tomomori-Sato, S. Sato, R. C. Conaway, J. W. Conaway, F. J. Asturias, Cell 2014, 157, 1430–1444;
- 37hK.-L. Tsai, S. Sato, C. Tomomori-Sato, R. C. Conaway, J. W. Conaway, F. J. Asturias, Nat. Struct. Mol. Biol. 2013, 20, 611–619;
- 37iX. Wang, Q. Sun, Z. Ding, J. Ji, J. Wang, X. Kong, J. Yang, G. Cai, Cell Res. 2014, 24, 796–808.
- 38
- 38aA. Meinhart, T. Kamenski, S. Hoeppner, S. Baumli, P. Cramer, Genes Dev. 2005, 19, 1401–1415;
- 38bM. Heidemann, C. Hintermair, K. Voß, D. Eick, Biochim. Biophys. Acta Gene Regul. Mech. 2013, 1829, 55–62;
- 38cC. Jeronimo, A. R. Bataille, F. Robert, Chem. Rev. 2013, 113, 8491–8522.
- 39
- 39aL. Larivière, M. Seizl, P. Cramer, Curr. Opin. Cell Biol. 2012, 24, 305–313;
- 39bZ. C. Poss, C. C. Ebmeier, D. J. Taatjes, Biochim. Biophys. Acta 2013, 48, 575–608;
- 39cC. Plaschka, K. Nozawa, P. Cramer, J. Mol. Biol. 2016, 428, 2569–2574.
- 40
- 40aY. Takagi, G. Calero, H. Komori, J. A. Brown, A. H. Ehrensberger, A. Hudmon, F. Asturias, R. D. Kornberg, Mol. Cell 2006, 23, 355–364;
- 40bL. Larivière, C. Plaschka, M. Seizl, L. Wenzeck, F. Kurth, P. Cramer, Nature 2012, 492, 448–451.
- 41T. Koschubs, K. Lorenzen, S. Baumli, S. Sandstrom, A. J. R. Heck, P. Cramer, Nucleic Acids Res. 2010, 38, 3186–3195.
- 42M. A. Cevher, Y. Shi, D. Li, B. T. Chait, S. Malik, R. G. Roeder, Nat. Struct. Mol. Biol. 2014, 21, 1028–1034.
- 43
- 43aT. Imasaki, G. Calero, G. Cai, K.-L. Tsai, K. Yamada, F. Cardelli, H. Erdjument-Bromage, P. Tempst, I. Berger, G. L. Kornberg, F. J. Asturias, R. D. Kornberg, Y. Takagi, Nature 2011, 475, 240–243;
- 43bP. J. J. Robinson, D. A. Bushnell, M. J. Trnka, A. L. Burlingame, R. D. Kornberg, Proc. Natl. Acad. Sci. USA 2012, 109, 17931–17935.
- 44T. Borggrefe, X. Yue, Semin. Cell Dev. Biol. 2011, 22, 759–768.
- 45
- 45aD. J. Taatjes, A. M. Na, E. Nogales, R. Tjian, Science 2002, 295, 1058–1062;
- 45bC. C. Ebmeier, D. J. Taatjes, Proc. Natl. Acad. Sci. USA 2010, 107, 11283–11288;
- 45cK. D. Meyer, S.-C. Lin, C. Bernecky, Y. Gao, D. J. Taatjes, Nat. Struct. Mol. Biol. 2010, 17, 753–760.
- 46
- 46aA. C. M. Cheung, P. Cramer, Cell 2012, 149, 1431–1437;
- 46bF. W. Martinez-Rucobo, P. Cramer, Biochim. Biophys. Acta Gene Regul. Mech. 2013, 1829, 9–19.
- 47
- 47aA. Hirata, B. J. Klein, K. S. Murakami, Nature 2008, 451, 851–854;
- 47bY. Korkhin, U. M. Unligil, O. Littlefield, P. J. Nelson, D. I. Stuart, P. B. Sigler, S. D. Bell, N. G. A. Abrescia, PLoS Biol. 2009, 7, e 1000102;
- 47cN. J. Proudfoot, Science 2016, 352, aad 9926;
- 47dV. Svetlov, E. Nudler, Biochim. Biophys. Acta Gene Regul. Mech. 2013, 1829, 20–28;
- 47eD. G. Vassylyev, S.-i. Sekine, O. Laptenko, J. Lee, M. N. Vassylyeva, S. Borukhov, S. Yokoyama, Nature 2002, 417, 712–719.
- 48
- 48aK. D. Westover, D. A. Bushnell, R. D. Kornberg, Cell 2004, 119, 481–489;
- 48bH. Kettenberger, K. J. Armache, P. Cramer, Mol. Cell 2004, 16, 955–965;
- 48cD. Wang, D. A. Bushnell, K. D. Westover, C. D. Kaplan, R. D. Kornberg, Cell 2006, 127, 941–954;
- 48dJ. F. Sydow, F. Brueckner, A. C. M. Cheung, G. E. Damsma, S. Dengl, E. Lehmann, D. Vassylyev, P. Cramer, Mol. Cell 2009, 34, 710–721;
- 48eA. C. M. Cheung, S. Sainsbury, P. Cramer, EMBO J. 2011, 30, 4755–4763.
- 49
- 49aD. G. Vassylyev, M. N. Vassylyeva, J. Zhang, M. Palangat, I. Artsimovitch, R. Landick, Nature 2007, 448, 163–168;
- 49bD. G. Vassylyev, M. N. Vassylyeva, A. Perederina, T. H. Tahirov, I. Artsimovitch, Nature 2007, 448, 157–162.
- 50T. A. Steitz, S. J. Smerdon, J. Jäger, C. M. Joyce, Science 1994, 266, 2022–2025.
- 51
- 51aD. A. Bushnell, P. Cramer, R. D. Kornberg, Proc. Natl. Acad. Sci. USA 2002, 99, 1218–1222;
- 51bC. D. Kaplan, K. M. Larsson, R. D. Kornberg, Mol. Cell 2008, 30, 547–556;
- 51cF. Brueckner, P. Cramer, Nat. Struct. Mol. Biol. 2008, 15, 811–818.
- 52J. F. Sydow, P. Cramer, Curr. Opin. Struct. Biol. 2009, 19, 732–739.
- 53N. Zenkin, Science 2006, 313, 518–520.
- 54A. C. M. Cheung, P. Cramer, Nature 2011, 471, 249–253.
- 55M. G. Izban, D. S. Luse, J. Biol. Chem. 1993, 268, 12864–12873.
- 56
- 56aH. Kettenberger, K. J. Armache, P. Cramer, Cell 2003, 114, 347–357;
- 56bD. Wang, D. Bushnell, X. Huang, K. Westover, M. Levitt, R. Kornberg, Cell 2009, 119, 481–489.
- 57G. E. Damsma, P. Cramer, J. Biol. Chem. 2009, 284, 31658–31663.
- 58L. Wang, Y. Zhou, L. Xu, R. Xiao, X. Lu, L. Chen, J. Chong, H. Li, C. He, X.-D. Fu, D. Wang, Nature 2015, 523, 621–625.
- 59
- 59aP. C. Hanawalt, G. Spivak, Nat. Rev. Mol. Cell Biol. 2008, 9, 958–970;
- 59bW. Vermeulen, M. Fousteri, Cold Spring Harbor Perspect. Biol. 2013, 5, a 012625.
- 60
- 60aF. Brueckner, U. Hennecke, T. Carell, P. Cramer, Science 2007, 315, 859–862;
- 60bG. E. Damsma, A. Alt, F. Brueckner, T. Carell, P. Cramer, Nat. Struct. Mol. Biol. 2007, 14, 1127–1133;
- 60cC. Walmacq, A. C. M. Cheung, M. L. Kireeva, L. Lubkowska, C. Ye, D. Gotte, J. N. Strathern, T. Carell, P. Cramer, M. Kashlev, Mol. Cell 2012, 46, 18–29.
- 61M. L. Kireeva, Y. A. Nedialkov, G. H. Cremona, Y. A. Purtov, L. Lubkowska, F. Malagon, Z. F. Burton, J. N. Strathern, M. Kashlev, Mol. Cell 2008, 30, 557–566.
- 62
- 62aF. Werner, J. Mol. Biol. 2012, 417, 13–27;
- 62bG. A. Hartzog, J. Fu, Biochim. Biophys. Acta Gene Regul. Mech. 2013, 1829, 105–115.
- 63
- 63aM. Guo, F. Xu, J. Yamada, T. Egelhofer, Y. Gao, G. A. Hartzog, M. Teng, L. Niu, Structure 2008, 16, 1649–1658;
- 63bA. Hirtreiter, G. E. Damsma, A. C. M. Cheung, D. Klose, D. Grohmann, E. Vojnic, A. C. R. Martin, P. Cramer, F. Werner, Nucleic Acids Res. 2010, 38, 4040–4051;
- 63cS. Wenzel, B. M. Martins, P. Rösch, B. M. Wöhrl, Biochem. J. 2010, 425, 373–380.
- 64
- 64aB. J. Klein, D. Bose, K. J. Baker, Z. M. Yusoff, X. Zhang, K. S. Murakami, Proc. Natl. Acad. Sci. USA 2011, 108, 546–550;
- 64bF. W. Martinez-Rucobo, S. Sainsbury, A. C. M. Cheung, P. Cramer, EMBO J. 2011, 30, 1302–1310.
- 65
- 65aJ. N. Kuehner, E. L. Pearson, C. Moore, Nat. Rev. Mol. Cell Biol. 2011, 12, 283–294;
- 65bH. E. Mischo, N. J. Proudfoot, Biochim. Biophys. Acta Gene Regul. Mech. 2013, 1829, 174–185.
- 66B. Schwalb, M. Michel, B. Zacher, K. Frühauf, C. Demel, A. Tresch, J. Gagneur, P. Cramer, Science 2016, 352, 1225–1228.
- 67J. Logan, E. Falck-Pedersen, J. E. Darnell, T. Shenk, Proc. Natl. Acad. Sci. USA 1987, 84, 8306–8310.
- 68
- 68aS. Connelly, J. L. Manley, Genes Dev. 1988, 2, 440–452;
- 68bN. J. Proudfoot, Trends Biochem. Sci. 1989, 14, 105–110.
- 69
- 69aS. West, N. Gromak, N. J. Proudfoot, Nature 2004, 432, 522–525;
- 69bM. Kim, N. J. Krogan, L. Vasiljeva, O. J. Rando, E. Nedea, J. F. Greenblatt, S. Buratowski, Nature 2004, 432, 517–522.
- 70
- 70aW. Luo, A. W. Johnson, D. L. Bentley, Genes Dev. 2006, 20, 954–965;
- 70bA. Schreieck, A. D. Easter, S. Etzold, K. Wiederhold, M. Lidschreiber, P. Cramer, L. A. Passmore, Nat. Struct. Mol. Biol. 2014, 21, 175–179.
- 71
- 71aS. H. Ahn, M. Kim, S. Buratowski, Mol. Cell 2004, 13, 67–76;
- 71bA. Mayer, M. Heidemann, M. Lidschreiber, A. Schreieck, M. Sun, C. Hintermair, E. Kremmer, D. Eick, P. Cramer, Science 2012, 336, 1723–1725.
- 72
- 72aH. Zhang, F. Rigo, H. G. Martinson, Mol. Cell 2015, 59, 437–448;
- 72bN. Fong, K. Brannan, B. Erickson, H. Kim, M. A. Cortazar, R. M. Sheridan, T. Nguyen, S. Karp, D. L. Bentley, Mol. Cell 2015, 60, 256–267.
- 73M. R. Paule, R. J. White, Nucleic Acids Res. 2000, 28, 1283–1298.
- 74A. Vannini, P. Cramer, Mol. Cell 2012, 45, 439–446.
- 75
- 75aC. Engel, S. Sainsbury, A. C. Cheung, D. Kostrewa, P. Cramer, Nature 2013, 502, 650–655;
- 75bC. Fernández-Tornero, M. Moreno-Morcillo, U. J. Rashid, N. M. I. Taylor, F. M. Ruiz, T. Gruene, P. Legrand, U. Steuerwald, C. W. Müller, Nature 2013, 502, 644–649.
- 76N. A. Hoffmann, A. J. Jakobi, M. Moreno-Morcillo, S. Glatt, J. Kosinski, W. J. H. Hagen, C. Sachse, C. W. Müller, Nature 2015, 528, 231–236.
- 77
- 77aC. Fernández-Tornero, B. Böttcher, U. J. Rashid, U. Steuerwald, B. Flörchinger, D. P. Devos, D. Lindner, C. W. Müller, EMBO J. 2010, 29, 3762–3772;
- 77bS. R. Geiger, K. Lorenzen, A. Schreieck, P. Hanecker, D. Kostrewa, A. J. R. Heck, P. Cramer, Mol. Cell 2010, 39, 583–594;
- 77cC.-D. Kuhn, S. R. Geiger, S. Baumli, M. Gartmann, J. Gerber, S. Jennebach, T. Mielke, H. Tschochner, R. Beckmann, P. Cramer, Cell 2007, 131, 1260–1272;
- 77dS. Lefèvre, H. Dumay-Odelot, L. El-Ayoubi, A. Budd, P. Legrand, N. Pinaud, M. Teichmann, S. Fribourg, Nat. Struct. Mol. Biol. 2011, 18, 352–358;
- 77eW. Ruan, E. Lehmann, M. Thomm, D. Kostrewa, P. Cramer, J. Biol. Chem. 2011, 286, 18701–18707;
- 77fA. Vannini, R. Ringel, A. G. Kusser, O. Berninghausen, G. A. Kassavetis, P. Cramer, Cell 2010, 143, 59–70;
- 77gC. C. Wu, F. Herzog, S. Jennebach, Y. C. Lin, C. Y. Pai, R. Aebersold, P. Cramer, H. T. Chen, Proc. Natl. Acad. Sci. USA 2012, 109, 19232–19237;
- 77hC. C. Wu, Y. C. Lin, H. T. Chen, Mol. Cell. Biol. 2011, 31, 2715–2728.
- 78A. Vannini, Biochim. Biophys. Acta Gene Regul. Mech. 2013, 1829, 258–264.
- 79
- 79aB. A. Knutson, S. Hahn, Science 2011, 333, 1637–1640;
- 79bS. Naidu, J. K. Friedrich, J. Russell, J. C. B. M. Zomerdijk, Science 2011, 333, 1640–1642;
- 79cA. López-De-León, M. Librizzi, K. Puglia, I. M. Willis, Cell 1992, 71, 211–220;
- 79dT. Colbert, S. Hahn, Genes Dev. 1992, 6, 1940–1949.
- 80K. J. Armache, S. Mitterweger, A. Meinhart, P. Cramer, J. Biol. Chem. 2005, 280, 7131–7134.