N−N Bond Forming Reductive Elimination via a Mixed-Valent Nickel(II)–Nickel(III) Intermediate
Justin B. Diccianni
Chemistry Department, New York University, 100 Washington Square E., New York, NY, 10003 USA
Search for more papers by this authorDr. Chunhua Hu
Chemistry Department, New York University, 100 Washington Square E., New York, NY, 10003 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Tianning Diao
Chemistry Department, New York University, 100 Washington Square E., New York, NY, 10003 USA
Search for more papers by this authorJustin B. Diccianni
Chemistry Department, New York University, 100 Washington Square E., New York, NY, 10003 USA
Search for more papers by this authorDr. Chunhua Hu
Chemistry Department, New York University, 100 Washington Square E., New York, NY, 10003 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Tianning Diao
Chemistry Department, New York University, 100 Washington Square E., New York, NY, 10003 USA
Search for more papers by this authorGraphical Abstract
Abstract
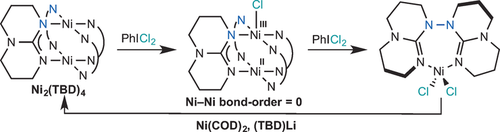
Natural products containing N–N bonds exhibit important biological activity. Current methods for constructing N−N bonds have limited scope. An advanced understanding of the fundamental N−N bond formation/cleavage processes occurring at the transition-metal center would facilitate the development of catalytic reactions. Herein we present an N−N bond-forming reductive elimination, which proceeds via a mixed-valent NiII–NiIII intermediate with a Ni–Ni bond order of zero. The discrete NiII–NiIII oxidation states contrast with the cationic dimeric Ni analogue, in which both Ni centers are equivalent with an oxidation state of 2.5. The electronic structures of these mixed-valent complexes have implications for the fundamental understanding of metal–metal bonding interactions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201602566-sup-0001-misc_information.pdf15.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. M. Blair, J. Sperry, J. Nat. Prod. 2013, 76, 794.
- 2Q. Zhang, A. Mándi, S. Li, Y. Chen, W. Zhang, X. Tian, H. Zhang, H. Li, W. Zhang, S. Zhang, J. Ju, T. Kurtán, C. Zhang, Eur. J. Org. Chem. 2012, 5256.
- 3
- 3aR. J. Parry, J. V. Mueller, J. Am. Chem. Soc. 1984, 106, 5764;
- 3bR. J. Parry, Y. Li, F. L. Lii, J. Am. Chem. Soc. 1992, 114, 10062;
- 3cT. Tao, L. B. Alemany, R. J. Parry, Org. Lett. 2003, 5, 1213.
- 4For leading references of hydrazine synthesis from amines, see:
- 4aR. Adams, B. K. Brown, Org. Synth. Coll. Vol. 1941, 1, 309;
- 4bJ.-P. Schirmann, P. Bourdauducq in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000.
- 5
- 5aT. Onaka, Tetrahedron Lett. 1968, 9, 5711;
10.1016/S0040-4039(00)70758-1 Google Scholar
- 5bC. C. Hughes, D. Trauner, Angew. Chem. Int. Ed. 2002, 41, 4556;
10.1002/1521-3773(20021202)41:23<4556::AID-ANIE4556>3.0.CO;2-E CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 4738;
- 5cM. Pangerl, C. C. Hughes, D. Trauner, Tetrahedron 2010, 66, 6626;
- 5dL. E. Evans, M. D. Cheeseman, K. Jones, Org. Lett. 2012, 14, 3546;
- 5eZ. Zheng, S. Ma, L. Tang, D. Zhang-Negrerie, Y. Du, K. Zhao, J. Org. Chem. 2014, 79, 4687.
- 6B. R. Rosen, E. W. Werner, A. G. O'Brien, P. S. Baran, J. Am. Chem. Soc. 2014, 136, 5571.
- 7
- 7aK. Kinoshita, Bull. Chem. Soc. Jpn. 1959, 32, 777;
- 7bL. Zhang, J. Xia, Q. Li, X. Li, S. W. Wang, Organometallics 2011, 30, 375;
- 7cC. B. R. Reddy, S. R. Reddy, S. Naidu, Catal. Commun. 2014, 56, 50.
- 8
- 8aF. Ragaini, A. Penoni, E. Gallo, S. Tollari, C. Li Gotti, M. Lapadula, E. Mangioni, S. Cenini, Chem. Eur. J. 2003, 9, 249;
- 8bR. A. Zarkesh, J. W. Ziller, A. F. Heyduk, Angew. Chem. Int. Ed. 2008, 47, 4715; Angew. Chem. 2008, 120, 4793;
- 8cK. J. Blackmore, N. Lal, J. W. Ziller, A. F. Heyduk, J. Am. Chem. Soc. 2008, 130, 2728;
- 8dN. D. Harrold, R. Waterman, G. L. Hillhouse, T. R. Cundari, J. Am. Chem. Soc. 2009, 131, 12872;
- 8eN. P. Mankad, P. Müller, J. C. Peters, J. Am. Chem. Soc. 2010, 132, 4083;
- 8fM. I. Lipschutz, T. D. Tilley, Chem. Commun. 2012, 48, 7146;
- 8gR. F. Munhá, R. A. Zarkesh, A. F. Heyduk, Dalton Trans. 2013, 42, 3751;
- 8hS. Vaddypally, S. K. Kondaveeti, J. H. Roudebush, R. J. Cava, M. J. Zdilla, Chem. Commun. 2014, 50, 1061;
- 8iJ. A. Bellow, M. Yousif, A. C. Cabelof, R. L. Lord, S. Groysman, Organometallics 2015, 34, 2917.
- 9For selective examples of catalytic azobenzene synthesis, see:
- 9aA. Grirrane, A. Corma, H. García, Science 2008, 322, 1661;
- 9bC. Zhang, N. Jiao, Angew. Chem. Int. Ed. 2010, 49, 6174; Angew. Chem. 2010, 122, 6310;
- 9cA. Takaoka, M.-E. Moret, J. C. Peters, J. Am. Chem. Soc. 2012, 134, 6695.
- 10For leading references, see:
- 10aL. A. Trimble, J. C. Vederas, J. Am. Chem. Soc. 1986, 108, 6397;
- 10bM. J. Burk, J. E. Feaster, J. Am. Chem. Soc. 1992, 114, 6266;
- 10cB. Zhao, H. Du, S. Cui, Y. Shi, J. Am. Chem. Soc. 2010, 132, 3523;
- 10dY. Zhu, R. G. Cornwall, H. Du, B. Zhao, Y. Shi, Acc. Chem. Res. 2014, 47, 3665.
- 11For representative examples, see:
- 11aC. E. Laplaza, C. C. Cummins, Science 1995, 268, 861;
- 11bD. J. Knobloch, E. Lobkovsky, P. J. Chirik, Nat. Chem. 2010, 2, 30;
- 11cK. Arashiba, Y. Miyake, Y. Nishibayashi, Nat. Chem. 2011, 3, 120;
- 11dM. M. Rodriguez, E. Bill, W. W. Brennessel, P. L. Holland, Science 2011, 334, 780.
- 12S. Z. Tasker, E. A. Standley, T. F. Jamison, Nature 2014, 509, 299.
- 13For leading precedents of Ni-mediated C−O and C−N bond formation, see:
- 13aP. T. Matsunaga, G. L. Hillhouse, A. L. Rheingold, J. Am. Chem. Soc. 1993, 115, 2075;
- 13bK. Koo, G. L. Hillhouse, Organometallics 1995, 14, 4421;
- 13cB. L. Lin, C. R. Clough, G. L. Hillhouse, J. Am. Chem. Soc. 2002, 124, 2890.
- 14
- 14aF. A. Cotton, M. Matusz, R. Poli, X. Feng, J. Am. Chem. Soc. 1988, 110, 1144;
- 14bC. Lin, J. D. Protasiewicz, T. Ren, Inorg. Chem. 1996, 35, 7455;
- 14cJ. F. Berry, F. A. Cotton, L. M. Daniels, C. A. Murillo, J. Am. Chem. Soc. 2002, 124, 3212;
- 14dJ. F. Berry, E. Bothe, F. A. Cotton, S. A. Ibragimov, C. A. Murillo, D. Villagrán, X. Wang, Inorg. Chem. 2006, 45, 4396.
- 15For selected examples, see:
- 15aF. A. Cotton, J. Gu, C. A. Murillo, D. J. Timmons, J. Am. Chem. Soc. 1998, 120, 13280;
- 15bF. A. Cotton, C. A. Murillo, X. Wang, C. C. Wilkinson, Inorg. Chim. Acta 2003, 351, 191;
- 15cF. A. Cotton, C. A. Murillo, J. H. Reibenspies, D. Villagrán, X. Wang, C. C. Wilkinson, Inorg. Chem. 2004, 43, 8373;
- 15dJ. F. Berry, F. A. Cotton, P. Huang, C. A. Murillo, X. Wang, Dalton Trans. 2005, 3713.
- 16G. J. Chuang, W. Wang, E. Lee, T. Ritter, J. Am. Chem. Soc. 2011, 133, 1760.
- 17
- 17aG. M. Chiarella, F. A. Cotton, C. Murillo, J. Cluster Sci. 2012, 23, 673;
- 17bF. A. Stokes, L. Kloo, P. J. Harford, A. J. Peel, R. J. Less, A. E. H. Wheatley, D. S. Wright, Aust. J. Chem. 2014, 67, 1081.
- 18See Supporting Information for additional data (Figure S7) and discussion about the mechanism of the observed C−N bond coupling.
- 19For a study of the NMR spectrum of a tetrahedral (diimine)NiCl2, see: P. Roquette, A. Maronna, M. Reinmuth, E. Kaifer, M. Enders, H.-J. Himmel, Inorg. Chem. 2011, 50, 1942.
- 20
- 20aV. A. Naumov, O. A. Litvinov, H. J. Geise, J. Dillen, J. Mol. Struct. 1983, 99, 303;
- 20bB. Ma, J.-H. Lii, K. Chen, N. L. Allinger, J. Phys. Chem. 1996, 100, 11297.
- 21K. A. Leñero, M. Kranenburg, Y. Guari, P. C. J. Kamer, P. W. N. M. van Leeuwen, S. Sabo-Etienne, B. Chaudret, Inorg. Chem. 2003, 42, 2859.
- 22For insertion of Ni(cod)2 into N−N bonds, see: A. G. Tskhovrebov, E. Solari, R. Scopelliti, K. Severin, Inorg. Chem. 2013, 52, 11688.
- 23S. T. Liddle, Molecular Metal-Metal Bonds: Compounds, Synthesis Properties, Wiley-VCH, Weinheim, 2015.
10.1002/9783527673353 Google Scholar
- 24For a study of intermolecular aggregation via 1H NMR spectroscopy as a function of sample concentration and temperature, see: A. Pérez, D. de Saá, A. Ballesteros, J. L. Serrano, T. Sierra, P. Romero, Chem. Eur. J. 2013, 19, 10271.
- 25DFT calculations were conducted using the ORCA package: F. Neese, Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73.
- 26The cationic [(TBD)4Ni2]+ is highly sensitive. Our efforts to grow single crystals were unsuccessful.
- 27Mixed-valent NiII–NiIII clusters have been reported, exhibiting an S=3/2 spin state:
- 27aT. Beissel, F. Birkelbach, E. Bill, T. Glaser, F. Kesting, C. Krebs, T. Weyhermüller, K. Wieghardt, C. Butzlaff, A. X. Trautwein, J. Am. Chem. Soc. 1996, 118, 12376;
- 27bB. Kersting, D. Siebert, Eur. J. Inorg. Chem. 1999, 189;
10.1002/(SICI)1099-0682(199901)1999:1<189::AID-EJIC189>3.0.CO;2-1 CAS Web of Science® Google Scholar
- 27cN. Kuwamura, K. i. Kitano, M. Hirotsu, T. Nishioka, Y. Teki, R. Santo, A. Ichimura, H. Hashimoto, L. J. Wright, I. Kinoshita, Chem. Eur. J. 2011, 17, 10708.
- 28D. C. Powers, T. Ritter, Nat. Chem. 2009, 1, 302.