Phosphoric Acid Catalyzed Desymmetrization of Bicyclic Bislactones Bearing an All-Carbon Stereogenic Center: Total Syntheses of (−)-Rhazinilam and (−)-Leucomidine B†
Jean-Baptiste Gualtierotti
Laboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304. 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Search for more papers by this authorDelphine Pasche
Laboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304. 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Search for more papers by this authorDr. Qian Wang
Laboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304. 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Search for more papers by this authorCorresponding Author
Prof. Dr. Jieping Zhu
Laboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304. 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Laboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304. 1015 Lausanne (Switzerland) http://lspn.epfl.chSearch for more papers by this authorJean-Baptiste Gualtierotti
Laboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304. 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Search for more papers by this authorDelphine Pasche
Laboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304. 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Search for more papers by this authorDr. Qian Wang
Laboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304. 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Search for more papers by this authorCorresponding Author
Prof. Dr. Jieping Zhu
Laboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304. 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Laboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304. 1015 Lausanne (Switzerland) http://lspn.epfl.chSearch for more papers by this authorWe thank EPFL (Switzerland), the Swiss National Science Foundation (SNSF), the Swiss National Centres of Competence in Research NCCR-Chemical Biology, the COST action (CM0905), and the Swiss State Secretariat for Education and Research for financial support.
Graphical Abstract
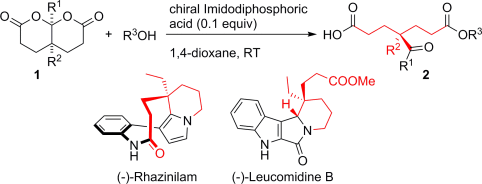
Breaking symmetry: Achiral bislactones (1) undergo desymmetrization by reaction with alcohol in the presence of chiral imidodiphosphoric acids. The monoacids 2, having an all-carbon stereogenic center, were obtained in good to excellent yields and enantioselectivities. Concise total syntheses of (−)-rhazinilam and (−)-leucomidine B were subsequently developed using 2 as a common starting material.
Abstract
In the presence of a catalytic amount of an imidodiphosphoric acid, enantioselective desymmetrization of bicyclic bislactones by reaction with alcohols took place smoothly to afford enantiomerically enriched monoacids having an all-carbon stereogenic center. Concise catalytic enantioselective syntheses of both (−)-rhazinilam and (−)-leucomidine B were subsequently developed using (S)-methyl 4-ethyl-4-formylpimelate monoacid as a common starting material.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201405842_sm_miscellaneous_information.pdf19.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. E. Kuehne, J. Am. Chem. Soc. 1964, 86, 2946.
- 2L. Castedo, J. Harley-Mason, M. Kaplan, J. Chem. Soc. D 1969, 1444.
- 3
- 3aJ.-Y. Laronze, J. Laronze-Fontaine, J. Lévy, J. Le Men, Tetrahedron Lett. 1974, 15, 491;
10.1016/S0040-4039(01)82252-8 Google Scholar
- 3bA. Nemes, C. Szántay, Jr., L. Czibula, I. Greiner, ARKIVOC 2008, 154.
- 4Aspidosperma family, see:
- 4aG. Costello, J. E. Saxton, Tetrahedron 1986, 42, 6047;
- 4bJ. W. Blowers, J. E. Saxton, A. G. Swanson, Tetrahedron 1986, 42, 6071; Attempted synthesis of rhazinilam, see:
- 4cC. Carité, J. P. Alazard, K. Ogino, C. Thal, Tetrahedron Lett. 1990, 31, 7011;
- 4dJ.-P. Alazard, C. Terrier, A. Mary, C. Thal, Tetrahedron 1994, 50, 6287.
- 5
- 5aM. Amat, M. Cantó, N. Llor, V. Ponzo, M. Pérez, J. Bosch, Angew. Chem. 2002, 114, 345;
10.1002/1521-3757(20020118)114:2<345::AID-ANGE345>3.0.CO;2-I Google ScholarAngew. Chem. Int. Ed. 2002, 41, 335;10.1002/1521-3773(20020118)41:2<335::AID-ANIE335>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 5bM. Amat, O. Bassas, N. Llor, M. Cantó, M. Pérez, E. Molins, J. Bosch, Chem. Eur. J. 2006, 12, 7872.
- 6J. C. F. Alves, A. B. C. Simas, P. R. R. Costa, J. D′Angelo, Tetrahedron: Asymmetry 1997, 8, 1963.
- 7
- 7aT. Gerfaud, C. Xie, L. Neuville, J. Zhu, Angew. Chem. 2011, 123, 4040;
10.1002/ange.201100257 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3954;
- 7bT. Buyck, Q. Wang, J. Zhu, Org. Lett. 2012, 14, 1338;
- 7cT. Buyck, Q. Wang, J. Zhu, Angew. Chem. 2013, 125, 12946;
10.1002/ange.201306663 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 12714;
- 7dZ. Xu, Q. Wang, J. Zhu, Angew. Chem. 2013, 125, 3354; Angew. Chem. Int. Ed. 2013, 52, 3272;
- 7eZ. Xu, Q. Wang, J. Zhu, J. Am. Chem. Soc. 2013, 135, 19127;
- 7fW. Ren, Q. Wang, J. Zhu, Angew. Chem. 2014, 126, 1849; Angew. Chem. Int. Ed. 2014, 53, 1818.
- 8
- 8aT. Akiyama, J. Itoh, K. Yokota, K. Fuchibe, Angew. Chem. 2004, 116, 1592;
10.1002/ange.200353240 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1566;
- 8bD. Uraguchi, K. Sorimatchi, M. Terada, J. Am. Chem. Soc. 2004, 126, 11804.
- 9
- 9aT. Akiyama, Chem. Rev. 2007, 107, 5744;
- 9bM. Terada, Chem. Commun. 2008, 4097;
- 9cD. Kampen, C. M. Reisinger, B. List, Top. Curr. Chem. 2010, 291, 395;
- 9dM. Terada, Curr. Org. Chem. 2011, 15, 2227;
- 9eM. Rueping, A. Kuenkel, I. Atodiresei, Chem. Soc. Rev. 2011, 40, 4539;
- 9fC. Zheng, S.-L. You, Chem. Soc. Rev. 2012, 41, 2498.
- 10E. García-Urdiales, I. Alfonso, V. Gotor, Chem. Rev. 2005, 105, 313.
- 11
- 11aY. Chen, P. McDavid, L. Deng, Chem. Rev. 2003, 103, 2965;
- 11bL. Atodiresei, I. Schiffers, C. Bolm, Chem. Rev. 2007, 107, 5683.
- 12Chiral phosphoric acid-catalyzed desymmetrization, see:
- 12aS. Xu, Z. Wang, X. Zhang, X. Zhang, K. Ding, Angew. Chem. 2008, 120, 2882; Angew. Chem. Int. Ed. 2008, 47, 2840;
- 12bE. B. Rowland, G. B. Rowland, E. Rivera-Otero, J. C. Antilla, J. Am. Chem. Soc. 2007, 129, 12084;
- 12cK. Mori, T. Katoh, T. Suzuki, T. Noji, M. Yamanaka, T. Akiyama, Angew. Chem. 2009, 121, 9832; Angew. Chem. Int. Ed. 2009, 48, 9652;
- 12dQ.-W. Zhang, C.-A. Fan, H.-J. Zhang, Y.-Q. Tu, Y.-M. Zhao, P. Gu, Z.-M. Chen, Angew. Chem. 2009, 121, 8724; Angew. Chem. Int. Ed. 2009, 48, 8572;
- 12eG. D. Sala, A. Lattanzi, Org. Lett. 2009, 11, 3330;
- 12fV. N. Wakchaure, B. List, Angew. Chem. 2010, 122, 4230;
10.1002/ange.201000637 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 4136;
- 12gQ. Gu, Z.-Q. Rong, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2010, 132, 4056;
- 12hL. Ren, T. Lei, L.-Z. Gong, Chem. Commun. 2011, 47, 11683;
- 12iS. Müller, M. J. Webber, B. List, J. Am. Chem. Soc. 2011, 133, 18534;
- 12jM. Yang, Y.-M. Zhao, S.-Y. Zhang, Y.-Q. Tu, F.-M. Zhang, Chem. Asian J. 2011, 6, 1344;
- 12kD. M. Rubush, M. A. Morges, B. J. Rose, D. H. Thamm, T. Rovis, J. Am. Chem. Soc. 2012, 134, 13554;
- 12lK. Mori, Y. Ichikawa, M. Kobayashi, Y. Shibata, M. Yamanaka, T. Akiyama, J. Am. Chem. Soc. 2013, 135, 3964;
- 12mZ. Wang, Z. Chen, J. Sun, Angew. Chem. 2013, 125, 6817; Angew. Chem. Int. Ed. 2013, 52, 6685;
- 12nZ. Chen, J. Sun, Angew. Chem. 2013, 125, 13838; Angew. Chem. Int. Ed. 2013, 52, 13593;
- 12oG. Qabaja, J. E. Wilent, A. R. Benavides, G. E. Bullard, K. S. Petersen, Org. Lett. 2013, 15, 1266;
- 12pA. D. Lackner, A. V. Samant, F. D. Toste, J. Am. Chem. Soc. 2013, 135, 14090; metal/phosphoric acid catalyzed desymmetrization:
- 12qZ. Chai, T. J. Rainey, J. Am. Chem. Soc. 2012, 134, 3615;
- 12rB. Guo, G. Schwarzwalder, J. T. Njardarson, Angew. Chem. 2012, 124, 5773; Angew. Chem. Int. Ed. 2012, 51, 5675;
- 12sA. K. Mourad, J. Leutzow, C. Czekelius, Angew. Chem. 2012, 124, 11311;
10.1002/ange.201205416 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11149.
- 13For other recent examples of chiral phosphoric acid catalyzed kinetic resolution/dynamic kinetic resolution, see:
- 13aF.-L. Sun, X.-J. Zheng, Q. Gu, Q.-L. He, S.-L. You, Eur. J. Org. Chem. 2010, 47;
- 13bM. Rueping, U. Uria, M.-Y. Lin, I. Atodiresei, J. Am. Chem. Soc. 2011, 133, 3732;
- 13cC. Wang, H.-W. Luo, L.-Z. Gong, Synlett 2011, 992;
- 13dG. Lu, V. B. Birman, Org. Lett. 2011, 13, 356;
- 13eM. Yamanaka, M. Hoshino, T. Katoh, K. Mori, T. Akiyama, Eur. J. Org. Chem. 2012, 4508;
- 13fX.-S. Wu, S.-K. Tian, Chem. Commun. 2012, 48, 898;
- 13gI. Čorić, J. H. Kim, T. Vlaar, M. Patil, W. Thiel, B. List, Angew. Chem. 2013, 125, 3574;
10.1002/ange.201209983 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 3490;
- 13hS. Harada, S. Kuwano, Y. Yamaoka, K.-I. Yamada, K. Takasu, Angew. Chem. 2013, 125, 10417;
10.1002/ange.201304281 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 10227;
- 13iK. Saito, Y. Shibata, M. Yamanaka, T. Akiyama, J. Am. Chem. Soc. 2013, 135, 11740; silver phosphate catalyzed:
- 13jY. Wang, K. Zheng, R. Hong, J. Am. Chem. Soc. 2012, 134, 4096.
- 14R. Chong, P. S. Clezy, Aust. J. Chem. 1967, 20, 123.
- 15CCDC 1002333 (12 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 16The structure of the bislactone 12 a in the solid state did not possess an obvious plane of symmetry. However, we use the term desymmetrization in this paper for the sake of convenience.
- 17
- 17aI. Čorić, B. List, Nature 2012, 483, 315;
- 17bS. Liao, I. Čorić, Q. Wang, B. List, J. Am. Chem. Soc. 2012, 134, 10765;
- 17cJ. H. Kim, I. Čorić, S. Vellalath, B. List, Angew. Chem. 2013, 125, 4570; Angew. Chem. Int. Ed. 2013, 52, 4474.
- 18
- 18aI. Čorić, S. Müller, B. List, J. Am. Chem. Soc. 2010, 132, 17370;
- 18bF. Xu, D. Huang, C. Han, W. Shen, X. Lin, Y. Wang, J. Org. Chem. 2010, 75, 8677.
- 19The quality of the monocrystal was not good enough to create a cif file. However, the so generated structure (see the Supporting Information) allowed us to determine without ambiguity the absolute configuration of 2 a.
- 20Isolation:
- 20aD. J. Abraham, R. D. Rosenstein, R. L. Lyon, H. H. S. Fong, Tetrahedron Lett. 1972, 13, 909;
10.1016/S0040-4039(01)84471-3 Google Scholar
- 20bK. T. De Silva, A. H. Ratcliffe, G. F. Smith, G. N. Smith, Tetrahedron Lett. 1972, 13, 913;
10.1016/S0040-4039(01)84472-5 Google Scholar
- 20cfor a review, see: O. Baudoin, D. Guénard, F. Guéritte, Mini-rev. Org. Chem. 2004, 1, 333.
- 21For total synthesis of (±)-rhazinilam, see:
- 21aA. H. Ratcliffe, G. F. Smith, G. N. Smith, Tetrahedron Lett. 1973, 14, 5179;
10.1016/S0040-4039(01)87657-7 Google Scholar
- 21bJ. A. Johnson, D. Sames, J. Am. Chem. Soc. 2000, 122, 6321;
- 21cP. Magnus, T. Rainey, Tetrahedron 2001, 57, 8647;
- 21dA. L. Bowie, Jr., C. C. Hughes, D. Trauner, Org. Lett. 2005, 7, 5207;
- 21eL. McMurray, E. M. Beck, M. J. Gaunt, Angew. Chem. 2012, 124, 9422;
10.1002/ange.201204151 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 9288;
- 21fX. Sui, R. Zhu, G. Li, X, Ma, Z. Gu, J. Am. Chem. Soc. 2013, 135, 9318; for synthesis of (−)-rhazinilam, see:
- 21gJ. A. Johnson, N. Li, D. Sames, J. Am. Chem. Soc. 2002, 124, 6900;
- 21hZ. Liu, A. S. Wasmuth, S. G. Nelson, J. Am. Chem. Soc. 2006, 128, 10352;
- 21iM. G. Banwell, D. A. S. Beck, A. C. Willis, ARKIVOC 2006, 163;
- 21jZ. Gu, A. Zakarian, Org. Lett. 2010, 12, 4224;
- 21kK. Sugimoto, K. Toyoshima, S. Nonaka, K. Kotaki, H. Ueda, H. Tokuyama, Angew. Chem. 2013, 125, 7309;
10.1002/ange.201303067 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 7168.
- 22M. Motegi, A. E. Nugroho, Y. Hirasawa, T. Arai, A. H. A. Hadi, H. Morita, Tetrahedron Lett. 2012, 53, 1227.
- 23Y.-C. Wu, J. Zhu, J. Org. Chem. 2008, 73, 9522.
- 24K. C. Nicolaou, C. J. N. Mathison, T. Montagnon, Angew. Chem. 2003, 115, 4211;
10.1002/ange.200352076 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4077.
- 25R. Grigg, P. Myers, A. Somasunderam, V. Sridharan, Tetrahedron 1992, 48, 9735.
- 26K. C. Nicolaou, A. A. Estrada, G. C. Freestone, S. H. Lee, X. Alvarez-Mico, Tetrahedron 2007, 63, 6088.
- 27Purification by semipreparative SFC on a chiral OD-H column afforded enantiopure leucomidine B.