Stereocontrolled Synthesis of 1,5-Stereogenic Centers through Three-Carbon Homologation of Boronic Esters†
Phillip J. Unsworth
School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS (UK)
Search for more papers by this authorDr. Daniele Leonori
School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS (UK)
Search for more papers by this authorCorresponding Author
Prof. Varinder K. Aggarwal
School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS (UK)
School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS (UK)Search for more papers by this authorPhillip J. Unsworth
School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS (UK)
Search for more papers by this authorDr. Daniele Leonori
School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS (UK)
Search for more papers by this authorCorresponding Author
Prof. Varinder K. Aggarwal
School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS (UK)
School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS (UK)Search for more papers by this authorWe thank the EPSRC-funded Bristol Chemical Synthesis Doctoral Training Centre for a Ph.D. studentship (P.J.U.) and Inochem-Frontier Scientific for their generous donation of boronic acids and esters. We thank the EPSRC (EP/I038071/1) and the European Research Council (FP7/2007–2013, ERC grant no. 246785) for financial support.
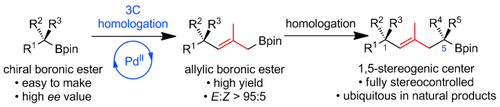
Graphical Abstract
Three more: The 3C homologation of chiral pinacol boronic esters gives di- or trisubstituted allylic boronic esters with high yield and E selectivities. The combination of this method with lithiation–borylation enables the synthesis of alkyl chains that bear 1,5-stereogenic centers. The utility of the process was demonstrated in a formal synthesis of (+)-jasplakinolide.
Abstract
Allylic pinacol boronic esters are stable toward 1,3-borotropic rearrangement. We developed a PdII-mediated isomerization process that gives di- or trisubstituted allylic boronic esters with high E selectivity. The combination of this method with lithiation–borylation enables the synthesis of carbon chains that bear 1,5-stereogenic centers. The utility of this method has been demonstrated in a formal synthesis of (+)-jasplakinolide.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201405700_sm_miscellaneous_information.pdf12.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews on polyketide synthesis, see:
- 1aK.-S. Yeung, I. Paterson, Chem. Rev. 2005, 105, 4237–4313;
- 1bA. Lorente, J. Lamariano-Merketegi, F. Albericio, M. Álvarez, Chem. Rev. 2013, 113, 4567–4610; for a review on the synthesis of (+)-jasplakinolide and its analogues, see:
- 1cY. Y. Xu, C. Liu, Z. P. Liu, Curr. Org. Synth. 2013, 10, 67–89; for a review on the synthesis of α-tocopherol, see:
- 1dT. Netscher, Vitam. Horm. 2007, 76, 155–202.
- 2This is especially the case when RCM is employed. For a review, see: A. Fürstner, Science 2013, 341, 1229713. In the specific case of (+)-jasplakinolide, prepared by ring-closing metathesis, an approximately 1:1 mixture of E/Z isomers was obtained:
- 2aR. Tannert, T.-S. Hu, H.-D. Arndt, H. Waldmann, Chem. Commun. 2009, 1493–1495;
- 2bR. Tannert, L.-G. Milroy, B. Ellinger, T.-S. Hu, H.-D. Arndt, H. Waldmann, J. Am. Chem. Soc. 2010, 132, 3063–3077.
- 3
- 3aJ. L. Stymiest, G. Dutheuil, A. Mahmood, V. K. Aggarwal, Angew. Chem. 2007, 119, 7635–7638;
10.1002/ange.200702146 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7491–7494;
- 3bG. Dutheuil, M. P. Webster, P. A. Worthington, V. K. Aggarwal, Angew. Chem. 2009, 121, 6435–6437;
10.1002/ange.200901194 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6317–6319;
- 3cT. G. Elford, S. Nave, R. P. Sonawane, V. K. Aggarwal, J. Am. Chem. Soc. 2011, 133, 16798–16801;
- 3dC. J. Fletcher, K. M. P. Wheelhouse, V. K. Aggarwal, Angew. Chem. 2013, 125, 2563–2566; Angew. Chem. Int. Ed. 2013, 52, 2503–2506; for other examples of lithium–boron chemistry, see:
- 3eE. Beckmann, V. Desai, D. Hoppe, Synlett 2004, 2275;
- 3fE. Beckmann, D. Hoppe, Synthesis 2005, 217;
- 3gG. Besong, K. Jarowicki, P. J. Kocienski, E. Sliwinski, T. F. Boyle, Org. Biomol. Chem. 2006, 4, 2193;
- 3hP. R. Blakemore, S. P. Marsden, H. D. Vater, Org. Lett. 2006, 8, 773–776;
- 3iP. R. Blakemore, M. S. Burge, J. Am. Chem. Soc. 2007, 129, 3068–3069; for reviews on the homologation of organoboron compounds, see:
- 3jS. P. Thomas, R. M. French, V. Jheengut, V. K. Aggarwal, Chem. Rec. 2009, 9, 24–39;
- 3kD. S. Matteson, J. Org. Chem. 2013, 78, 10009–10023.
- 4H. C. Brown, M. V. Rangaishenvi, S. Jayaraman, Organometallics 1992, 11, 1948–1954.
- 51-Chloro allyllithium has been shown to be effective for the homologation of tertiary pinacol boronic esters:
- 5aR. P. Sonawane, V. Jheengut, C. Rabalakos, R. Larouche-Gauthier, H. K. Scott, V. K. Aggarwal, Angew. Chem. 2011, 123, 3844–3847;
10.1002/ange.201008067 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3760–3763; 3-chloro-1-lithiopropene (generated from the non-commercially available tin or bromo derivative) has recently been shown to be effective as homologating reagent for the synthesis of allylic boronic esters:
- 5bA. P. Pulis, D. J. Blair, E. Torres, V. K. Aggarwal, J. Am. Chem. Soc. 2013, 135, 16054–16057;
- 5cK. Smith, M. C. Elliott, D. H. Jones, J. Org. Chem. 2013, 78, 9526–9531.
- 6
- 6aY. N. Bubnov, Pure Appl. Chem. 1987, 59, 895;
- 6bY. N. Bubnov, M. E. Gurskii, I. D. Gridnev, A. V. Ignatenko, Y. A. Ustynyuk, V. I. Mstislavsky, J. Organomet. Chem. 1992, 424, 127–132.
- 7M. Lombardo, S. Morganti, M. Tozzi, C. Trombini, Eur. J. Org. Chem. 2002, 2823–2830.
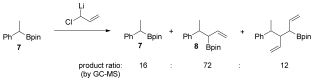
- 8The following inseparable mixture of products was observed when boronic ester 7 was treated with 1-chloro allyllithium:
- 9
- 9aD. S. Matteson, D. Majumdar, J. Am. Chem. Soc. 1980, 102, 7588–7590;
- 9bD. S. Matteson, R. Ray, J. Am. Chem. Soc. 1980, 102, 7590–7591.
- 10
- 10aT. Ishiyama, T.-a. Ahiko, N. Miyaura, Tetrahedron Lett. 1996, 37, 6889–6892;
- 10bT.-a. Ahiko, T. Ishiyama, N. Miyaura, Chem. Lett. 1997, 26, 811–812;
- 10cS. Sebelius, O. A. Wallner, K. J. Szabó, Org. Lett. 2003, 5, 3065–3068; for the Ni-catalyzed borylation of allylic carbonates, see:
- 10dP. Zhang, I. A. Roundtree, J. P. Morken, Org. Lett. 2012, 14, 1416–1419.
- 11
- 11aG. Dutheuil, N. Selander, K. J. Szabó, V. K. Aggarwal, Synthesis 2008, 2293–2297;
- 11bN. Selander, J. R. Paasch, K. J. Szabó, J. Am. Chem. Soc. 2011, 133, 409–411;
- 11cJ. M. Larsson, K. J. Szabó, J. Am. Chem. Soc. 2013, 135, 443–455.
- 12For one example of the E-selective synthesis of a β-methyl-substituted allylic boronic ester, see:
- 12aS. Roesner, C. A. Brown, M. Mohiti, A. P. Pulis, R. Rasappan, D. J. Blair, S. Essafi, D. Leonori, V. K. Aggarwal, Chem. Commun. 2014, 50, 4053–4055; for selected examples of Z-selective syntheses of β-methyl-substituted allylic boronic esters, see:
- 12bR. J. Ely, J. P. Morken, J. Am. Chem. Soc. 2010, 132, 2534–2535; for selected examples of E-selective syntheses of β-methyl-substituted allylic boronic esters that also contain an α-alkyl substituent, see:
- 12cM. J. Hesse, C. P. Butts, C. L. Willis, V. K. Aggarwal, Angew. Chem. 2012, 124, 12612–12616;
10.1002/ange.201207312 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12444–12448;
- 12dJ. L. Y. Chen, H. K. Scott, M. J. Hesse, C. L. Willis, V. K. Aggarwal, J. Am. Chem. Soc. 2013, 135, 5316–5319; for an example of the stereoselective synthesis of allenyl and propargyl boronates, see:
- 12eT. S. N. Zhao, Y. Yang, T. Lessing, K. J. Szabó, J. Am. Chem. Soc. 2014, 136, 7563–7566.
- 13For these boronic esters it was difficult to achieve a full conversion because of the precipitation of Pd black out of solution before the reaction was complete. To overcome this problem, an excess of CuCl2 was added and 1.5 equivalents of B2pin2 was sufficient under these conditions.
- 14
- 14aT. Ishiyama, M. Murata, N. Miyaura, J. Org. Chem. 1995, 60, 7508–7510;
- 14bK.-J. Chang, D. K. Rayabarapu, F.-Y. Yang, C.-H. Cheng, J. Am. Chem. Soc. 2005, 127, 126–131;
- 14cM. Daini, M. Suginome, Chem. Commun. 2008, 5224–5226;
- 14dM. Daini, M. Suginome, J. Am. Chem. Soc. 2011, 133, 4758–4761;
- 14eM. Daini, A. Yamamoto, M. Suginome, J. Am. Chem. Soc. 2008, 130, 2918–2919.
- 15For examples of the reaction of allylic boronic esters with Ar–PdII complexes, see:
- 15aB. Glasspoole, K. Ghozati, J. Moir, C. Crudden, Chem. Commun. 2012, 48, 1230–1232;
- 15bL. Chausset-Boissarie, K. Ghozati, E. LaBine, J.-K. Chen, V. K. Aggarwal, C. Crudden, Chem. Eur. J. 2013, 19, 17698–17701; for an example of the reaction of primary allylic boronic acids with Ar–PdII complexes, see:
- 15cS. Sebelius, V. J. Olsson, O. A. Wallner, K. Szabò, J. Am. Chem. Soc. 2006, 128, 8150–8151.
- 16Prepared by the asymmetric hydroboration of styrene: D. Noh, S. K. Yoon, J. Won, J. Y. Lee, J. Yun, Chem. Asian J. 2011, 6, 1967–1969.
- 17(+)-Sparteine is commercially available and was purchased from BOC Sciences.
- 18
- 18aK. M. Sadhu, D. S. Matteson, Organometallics 1985, 4, 1687–1689;
- 18bH. C. Brown, S. M. Singh, M. V. Rangaishenvi, J. Org. Chem. 1986, 51, 3150–3155.
- 19P. A. Grieco, Y. S. Hon, A. Perez-Medrano, J. Am. Chem. Soc. 1988, 110, 1630–1631.