Nickel-Catalyzed Cross-Coupling of Functionalized Difluoromethyl Bromides and Chlorides with Aryl Boronic Acids: A General Method for Difluoroalkylated Arenes†
Yu-Lan Xiao
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorWen-Hao Guo
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorGuo-Zhen He
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorQiang Pan
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Xingang Zhang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)Search for more papers by this authorYu-Lan Xiao
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorWen-Hao Guo
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorGuo-Zhen He
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorQiang Pan
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Xingang Zhang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)Search for more papers by this authorThis work was financially supported by the National Basic Research Program of China (973 Program) (No. 2012CB821600), the NSFC (21172242 and 21332010), and SIOC.
Graphical Abstract
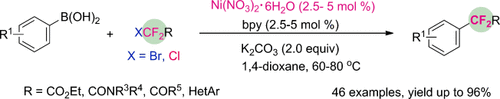
Simple and easy: The first example of a nickel-catalyzed difluoroalkylation of aryl boronic acids with functionalized difluoromethyl bromides and chlorides has been developed. This cross-coupling process features a broad substrate scope, a cheap catalyst, and excellent functional-group compatibility.
Abstract
Transition-metal-catalyzed difluoroalkylation of aromatics remains challenging despite the importance of difluoroalkylated arenes in medicinal chemistry. Herein, the first successful example of nickel-catalyzed difluoroalkylation of aryl boronic acids is described. The reaction allows access to a variety of functionalized difluoromethyl bromides and chlorides, and paves the way to highly cost-efficient synthesis of a wide range of difluoroalkylated arenes. The notable features of this protocol are its high generality, excellent functional-group compatibility, low-cost nickel-catalyst, and practicality for gram-scale production, thus providing a facile method for applications in drug discovery and development.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201405653_sm_miscellaneous_information.pdf6.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews, see:
- 1aB. E. Smart, J. Fluorine Chem. 2001, 109, 3;
- 1bP. Maienfisch, R. G. Hall, Chimia 2004, 58, 93;
- 1cSpecial issue on “Fluorine in the Life Sciences”, ChemBioChem 2004, 5, 557;
10.1002/cbic.200490021 Google Scholar
- 1dK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881;
- 1eS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320.
- 2For selected reviews, see:
- 2aT. Furuya, A. S. Kamlet, T. Ritter, Nature 2011, 473, 470;
- 2bO. A. Tomashenko, V. V. Grushin, Chem. Rev. 2011, 111, 4475;
- 2cC. Hollingworth, V. Gouverneur, Chem. Commun. 2012, 48, 2929;
- 2dT. Besset, C. Schneider, D. Cahard, Angew. Chem. 2012, 124, 5134;
10.1002/ange.201201012 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5048.
- 3T. Liang, C. N. Neumann, T. Ritter, Angew. Chem. 2013, 125, 8372; Angew. Chem. Int. Ed. 2013, 52, 8214.
- 4For transition-metal-mediated difluoroalkylation, see:
- 4aT. Taguchi, O. Kitagawa, T. Morikawa, T. Nishiwaki, H. Uehara, H. Endo, Y. Kobayashi, Tetrahedron Lett. 1986, 27, 6103;
- 4bW. Qiu, D. J. Burton, Tetrahedron Lett. 1996, 37, 2745;
- 4cT. Yokomatsu, T. Murano, K. Suemune, S. Shibuya, Tetrahedron 1997, 53, 815;
- 4dP. S. Fier, J. F. Hartwig, J. Am. Chem. Soc. 2012, 134, 5524;
- 4eG. K. S. Prakash, S. K. Ganesh, J.-P. Jones, A. Kulkarni, K. Masood, J. K. Swabeck, G. A. Olah, Angew. Chem. 2012, 124, 12256;
10.1002/ange.201205850 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12090;
- 4fY. Fujiwara, J. A. Dixon, R. A. Rodriguez, R. D. Baxter, D. D. Dixon, M. R. Collins, D. G. Blackmond, P. S. Baran, J. Am. Chem. Soc. 2012, 134, 1494.
- 5For transition-metal-catalyzed difluoroalkylation, see:
- 5aK. Fujikawa, Y. Fujioka, A. Kobayashi, H. Amii, Org. Lett. 2011, 13, 5560;
- 5bZ. Feng, F. Chen, X. Zhang, Org. Lett. 2012, 14, 1938;
- 5cZ. Feng, Y.-L. Xiao, X. Zhang, Org. Chem. Front. 2014, 1, 113;
- 5dZ. Feng, Q.-Q. Min, Y.-L. Xiao, B. Zhang, X. Zhang, Angew. Chem. 2014, 126, 1695; Angew. Chem. Int. Ed. 2014, 53, 1669;
- 5eQ.-Q. Min, Z. Yin, Z. Feng, W.-H. Guo, X. Zhang, J. Am. Chem. Soc. 2014, 136, 1230;
- 5fS. Ge, W. Chaladj, J. F. Hartwig, J. Am. Chem. Soc. 2014, 136, 4149;
- 5gC. Guo, R.-W. Wang, F.-L. Qing, J. Fluorine Chem. 2012, 143, 135.
- 6
- 6aC. M. Blackburn, D. A. England, F. Kolkmann, J. Chem. Soc. Chem. Commun. 1981, 930;
- 6bG. M. Blackburn, D. E. Kent, F. Kolkmann, J. Chem. Soc. Perkin Trans. 1 1984, 1119;
- 6cT. Kitazume, T. Kamazaki, Experimental Methods in Organic Fluorine Chemistry, Gordon and Breach Science, Tokyo, 1998.
- 7
- 7aJ. O. Link, J. G. Taylor, L. Xu, M. Mitchell, H. Guo, H. Liu, D. Kato, T. Kirschberg, J. Sun, N. Squires, J. Parrish, T. Keller, Z.-Y. Yang, C. Yang, M. Matles, Y. Wang, K. Wang, G. Cheng, Y. Tian, E. Mogalian, E. Mondou, M. Cornpropst, J. Perry, M. C. Desai, J. Med. Chem. 2014, 57, 2033;
- 7bJR. T. R. Burke, K. Lee, Acc. Chem. Res. 2003, 36, 426;
- 7cZ.-Y. Zhang, Acc. Chem. Res. 2003, 36, 385.
- 8
- 8aR. Eujen, B. Hoge, D. J. Brauer, J. Organomet. Chem. 1996, 519, 7;
- 8bT. Yokomatsu, K. Suemune, T. Murano, S. Shibuya, J. Org. Chem. 1996, 61, 7207;
- 8cD. J. Burton, G. A. Hartgrave, J. Fluorine Chem. 2007, 128, 1198.
- 9E. J. Cho, T. D. Senecal, T. Kinzel, Y. Zhang, D. A. Watson, S. L. Buchwald, Science 2010, 328, 1679.
- 10M. Oishi, H. Kondo, H. Amii, Chem. Commun. 2009, 1909.
- 11For nickel(0)-catalyzed free radical perfluoroalkylations of aromatics, see:
- 11aQ.-L. Zhou, Y.-Z. Huang, J. Fluorine Chem. 1989, 43, 385;
- 11bX.-T. Huang, Q.-Y. Chen, J. Org. Chem. 2001, 66, 4651; For Ni0-catalyzed difluoroacetylation of vinylzirconiums, see:
- 11cM. K. Schwaebe, J. R. McCarthy, J. P. Whitten, Tetrahedron Lett. 2000, 41, 791.
- 12
- 12aG. G. Dubinina, W. W. Brennessel, J. L. Miller, D. A. Vicic, Organometallics 2008, 27, 3933;
- 12bA. Klein, D. A. Vicic, C. Biewer, I. Kieltsch, K. Stirnat, C. Hamacher, Organometallics 2012, 31, 5334;
- 12cC.-P. Zhang, H. Wang, A. Klein, C. Biewer, K. Stirnat, Y. Yamaguchi, L. Xu, V. Gomez-Benitez, D. A. Vicic, J. Am. Chem. Soc. 2013, 135, 8141.
- 13During our manuscript preparation, a nickel-catalyzed asymmetric synthesis of tertiary alkyl fluorides between α-bromo-α-fluoroketones with aryl zinc reagents has been reported. See: Y. Liang, G. C. Fu, J. Am. Chem. Soc. 2014, 136, 5520.
- 14
- 14aX. Zhang, S. Fan, C.-Y. He, X. Wan, Q.-Q. Min, J. Yang, Z.-X. Jiang, J. Am. Chem. Soc. 2010, 132, 4506;
- 14bC.-Y. He, S. Fan, X. Zhang, J. Am. Chem. Soc. 2010, 132, 12850;
- 14cS. Fan, F. Chen, X. Zhang, Angew. Chem. 2011, 123, 6040; Angew. Chem. Int. Ed. 2011, 50, 5918.
- 15Bromodifluoromethylated benzoxazole, benzoimidazole, and thiazole can be easily prepared. See:
- 15aW. R. Dolbier Jr., C. R. Burkholder, M. Medebielle, J. Fluorine Chem. 1999, 95, 127;
- 15bH. Jiang, S. Yuan, Y. Cai, W. Wan, S. Zhu, J. Hao, J. Fluorine Chem. 2012, 133, 167.
- 16
- 16aZ. Kazimierczuk, M. Andrzejewska, J. Kaustova, V. Klimesova, Eur. J. Med. Chem. 2005, 40, 203;
- 16bF. Hernández-Luis, A. Hernández-Campos, R. Castillo, G. Navarrete-Vázquez, O. Soria-Arteche, M. Hernández-Hernández, L. Yépez-Mulia, Eur. J. Med. Chem. 2010, 45, 3135.
- 17R. C. Bernotas, J. M. Travins, J. E. Wrobel, D. H. Kaufman, WO 2009086138, 2009.
- 18For mechanistic studies of nickel-catalyzed Suzuki reactions of unactivated alkyl electrophiles, see:
- 18aA. Wilsily, F. Tramutola, N. A. Owston, G. C. Fu, J. Am. Chem. Soc. 2012, 134, 5794;
- 18bS. L. Zultanski, G. C. Fu, J. Am. Chem. Soc. 2013, 135, 624; For early mechanistic studies of nickel-catalyzed Negishi reactions of unactivated alkyl electrophiles, see:
- 18cG. D. Jones, J. L. Martin, C. McFarland, O. R. Allen, R. E. Hall, A. D. Haley, R. J. Brandon, T. Konovalova, P. J. Desrochers, P. Pulay, D. A. Vicic, J. Am. Chem. Soc. 2006, 128, 13175;
- 18dV. B. Phapale, E. Bunuel, M. Garcia-Iglesias, D. J. Cardenas, Angew. Chem. 2007, 119, 8946;
10.1002/ange.200702528 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8790;
- 18eX. Lin, D. L. Phillips, J. Org. Chem. 2008, 73, 3680; For an overview of mechanistic issues regarding nickel-catalyzed cross-couplings of unactivated alkyl halides, see:
- 18fX. Hu, Chem. Sci. 2011, 2, 1867.