Synthesis and Duplex-Stabilizing Properties of Fluorinated N-Methanocarbathymidine Analogues Locked in the C3′-endo Conformation†
Corresponding Author
Michael E. Jung
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095 (USA)
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095 (USA)Search for more papers by this authorTimothy A. Dwight
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095 (USA)
Search for more papers by this authorFrederic Vigant
Department of Microbiology, Immunology, and Molecular Genetics, University of California (USA)
Search for more papers by this authorMichael E. Østergaard
Department of Medicinal Chemistry, Isis Pharmaceuticals, 2855 Gazelle Court, Carlsbad, CA 92010 (USA)
Search for more papers by this authorEric E. Swayze
Department of Medicinal Chemistry, Isis Pharmaceuticals, 2855 Gazelle Court, Carlsbad, CA 92010 (USA)
Search for more papers by this authorPunit P. Seth
Department of Medicinal Chemistry, Isis Pharmaceuticals, 2855 Gazelle Court, Carlsbad, CA 92010 (USA)
Search for more papers by this authorCorresponding Author
Michael E. Jung
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095 (USA)
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095 (USA)Search for more papers by this authorTimothy A. Dwight
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095 (USA)
Search for more papers by this authorFrederic Vigant
Department of Microbiology, Immunology, and Molecular Genetics, University of California (USA)
Search for more papers by this authorMichael E. Østergaard
Department of Medicinal Chemistry, Isis Pharmaceuticals, 2855 Gazelle Court, Carlsbad, CA 92010 (USA)
Search for more papers by this authorEric E. Swayze
Department of Medicinal Chemistry, Isis Pharmaceuticals, 2855 Gazelle Court, Carlsbad, CA 92010 (USA)
Search for more papers by this authorPunit P. Seth
Department of Medicinal Chemistry, Isis Pharmaceuticals, 2855 Gazelle Court, Carlsbad, CA 92010 (USA)
Search for more papers by this authorM.E.J., T.A.D., and F.V. thank Isis Pharmaceuticals for the generous support of this research. We also thank Dr. Saeed Khan for the X-ray crystal structure and Prof. Miguel Garcia-Garibay for discussions. This material is based upon research supported by the National Science Foundation under equipment grant no. CHE-1048804.
Graphical Abstract
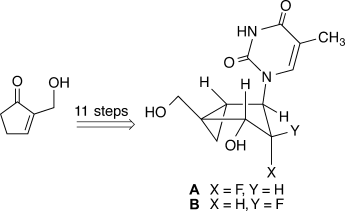
The power of conviction: The efficient synthesis, antiviral activity, and duplex-stabilizing properties of both isomers, A and B, of the 2′-fluoro analogue of N-methanocarbathymidine (N-MCT) are reported. Incorporation of the fluorine substituent at the 2′-position of the N-MCT scaffold was found to have a strong positive effect on duplex thermal stability.
Abstract
The efficient synthesis, antiviral activity, and duplex-stabilizing properties of both isomers of the 2′-fluoro analogue of Northern methanocarbathymidine (N-MCT), 2 and 3, are reported. We show that 2′-F incorporation on the N-MCT scaffold has a strong stabilizing effect on duplex thermal stability.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201405283_sm_miscellaneous_information.pdf12.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. W. Ng, D. T. Shima, P. Calias, E. T. Cunningham, Jr., D. R. Guyer, A. P. Adamis, Nat. Rev. Drug Discovery 2006, 5, 123–132;
- 1bW. Pieken, D. Olsen, F. Benseler, H. Aurup, F. Eckstein, Science 1991, 253, 314–317;
- 1cB. P. Monia, E. A. Lesnik, C. Gonzalez, W. F. Lima, D. McGee, C. J. Guinosso, A. M. Kawasaki, P. D. Cook, S. M. Freier, J. Biol. Chem. 1993, 268, 14514–14522;
- 1dF. Rigo, Y. Hua, S. J. Chun, T. P. Prakash, A. R. Krainer, C. F. Bennett, Nat. Chem. Biol. 2012, 8, 555–561;
- 1eM. Manoharan, A. Akinc, R. K. Pandey, J. Qin, P. Hadwiger, M. John, K. Mills, K. Charisse, M. A. Maier, L. Nechev, E. M. Greene, P. S. Pallan, E. Rozners, K. G. Rajeev, M. Egli, Angew. Chem. 2011, 123, 2332–2336; Angew. Chem. Int. Ed. 2011, 50, 2284–2288;
- 1fW. F. Lima, T. P. Prakash, H. M. Murray, G. A. Kinberger, W. Li, A. E. Chappell, C. S. Li, S. F. Murray, H. Gaus, P. P. Seth, E. E. Swayze, S. T. Crooke, Cell 2012, 150, 883–894;
- 1gS. Davis, S. Propp, S. M. Freier, L. E. Jones, M. J. Serra, G. Kinberger, B. Bhat, E. E. Swayze, C. F. Bennett, C. Esau, Nucleic Acids Res. 2009, 37, 70–77;
- 1hE. A. Véliz, L. M. Easterwood, P. A. Beal, J. Am. Chem. Soc. 2003, 125, 10867–10876.
- 2M. J. Sofia, W. Chang, P. A. Furman, R. T. Mosley, B. S. Ross, J. Med. Chem. 2012, 55, 2481–2531.
- 3M. A. Villalona-Calero, P. Ritch, J. A. Figueroa, G. A. Otterson, R. Belt, E. Dow, S. George, J. Leonardo, S. McCachren, G. L. Miller, M. Modiano, M. Valdivieso, R. Geary, J. W. Oliver, J. Holmlund, J. Clin. Cancer Res. 2004, 10, 6086–6093.
- 4P. Herdewijn, Chem. Biodiversity 2010, 7, 1–59.
- 5M. Egli, P. S. Pallan, C. R. Allerson, T. P. Prakash, A. Berdeja, J. Yu, S. Lee, A. Watt, H. Gaus, B. Bhat, E. E. Swayze, P. P. Seth, J. Am. Chem. Soc. 2011, 133, 16642–16649.
- 6P. P. Seth, J. Yu, A. Jazayeri, P. S. Pallan, C. R. Allerson, M. E. Østergaard, F. Liu, P. Herdewijn, M. Egli, E. E. Swayze, J. Org. Chem. 2012, 77, 5074–5085.
- 7
- 7aM. E. Jung, I. D. Trifunovich, J. M. Gardiner, G. L. Clevenger, J. Chem. Soc. Chem. Commun. 1990, 84–85;
- 7bM. E. Jung, J. M. Gardiner, J. Org. Chem. 1991, 56, 2614–2615;
- 7cM. E. Jung, C. Castro, J. M. Gardiner, Tetrahedron Lett. 1991, 32, 5717–5720;
- 7dM. E. Jung, I. D. Trifunovich, Tetrahedron Lett. 1992, 33, 2921–2924;
- 7eM. E. Jung, J. M. Gardiner, Tetrahedron Lett. 1992, 33, 3841–3844;
- 7fM. E. Jung, C. Castro, J. Org. Chem. 1993, 58, 807–808;
- 7gM. E. Jung, A. W. Sledeski, J. Chem. Soc. Chem. Commun. 1993, 589–591;
- 7hM. E. Jung, H. Rhee, Tetrahedron Lett. 1993, 34, 4449–4452;
- 7iM. E. Jung, H. Rhee, J. Org. Chem. 1994, 59, 4719–4720;
- 7jM. E. Jung, C. J. Nichols, Tetrahedron Lett. 1996, 37, 7667–7670.
- 8K.-H. Altmann, R. Kesselring, E. Francotte, G. Rihs, Tetrahedron Lett. 1994, 35, 2331–2334.
- 9
- 9aM. A. Siddiqui, H. Ford, Jr., C. George, V. E. Marquez, Nucleosides Nucleotides 1996, 15, 235–250;
- 9bV. E. Marquez, M. A. Siddiqui, A. Ezzitouni, P. Russ, J. Wang, R. W. Wagner, M. D. Matteucci, J. Med. Chem. 1996, 39, 3739–3747.
- 10
- 10aA. Ezzitouni, P. Russ, V. E. Marquez, J. Org. Chem. 1997, 62, 4870–4873;
- 10bV. E. Marquez, A. Ezzitouni, M. A. Siddiqui, P. Russ, H. Ikeda, C. George, Nucleosides Nucleotides 1997, 16, 1431–1434;
- 10cfor a review, see: V. E. Marquez, S. H. Hughes, S. Sei, R. Agbaria, Antiviral Res. 2006, 71, 268–275.
- 11A. Patra, M. Paolillo, K. Charisse, M. Manoharan, E. Rozners, M. Egli, Angew. Chem. 2012, 124, 12033–12036; Angew. Chem. Int. Ed. 2012, 51, 11863–11866.
- 12Compound 4 was prepared by the Morita–Baylis–Hillman reaction of cyclopentenone and formaldehyde in 97 % yield. H. Ito, Y. Takenaka, S. Fukunishi, K. Iguchi, Synthesis 2005, 3035–3038.
- 13
- 13aE. J. Corey, R. K. Bakshi, S. Shibata, J. Am. Chem. Soc. 1987, 109, 5551–5553;
- 13bE. J. Corey, R. K. Bakshi, S. Shibata, C. P. Chen, V. K. Singh, J. Am. Chem. Soc. 1987, 109, 7925–7926;
- 13cE. J. Corey, S. Shibata, R. K. Bakshi, J. Org. Chem. 1988, 53, 2861–2863;
- 13dE. J. Corey, C. J. Helal, Angew. Chem. 1998, 110, 2092–2118;
10.1002/(SICI)1521-3757(19980803)110:15<2092::AID-ANGE2092>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1986–2012.10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 14J. A. Dale, D. L. Dull, H. S. Mosher, J. Org. Chem. 1969, 34, 2543–2549.
- 15
- 15aV. E. Marquez, P. Wang, M. C. Nicklaus, M. Maier, M. Manoharan, J. K. Christman, N. K. Banavali, A. D. Mackerell, Jr., Nucleosides Nucleotides Nucleic Acids 2001, 20, 451–459;
- 15bP. Wang, A. S. Brank, N. K. Banavali, M. C. Nicklaus, V. E. Marquez, J. K. Christman, A. D. MacKerell, Jr., J. Am. Chem. Soc. 2000, 122, 12422–12434.
- 16The oxidation of 7 by other methods (e.g. Swern, PCC, IBX) gave 8 in lower yield. We also attempted to prepare the alcohol of 8 (i.e. without the TBS protecting group) from 4 directly by using the enantioselective Charette modification of the Simmons–Smith reaction with an optically active dioxaborolane, but did not observe any product. A. B. Charette, H. Juteau, J. Am. Chem. Soc. 1994, 116, 2651–2652.
- 17Y. Ito, T. Hirao, T. Saegusa, J. Org. Chem. 1978, 43, 1011–1013. The oxidative method to prepare this enone by using a selenoxide elimination did not proceed well in this case.
- 18The addition of azide to the α-fluoroenone 10 (TMSN3/AcOH/CH2C2; then Et3N) afforded the desired addition product as a mixture of diastereomers at the fluorine atom, but in only moderate yield (26 %).
- 19
- 19aM. J. Minch, Concepts Magn. Reson. 1994, 6, 41–56;
- 19bM. Karplus, J. Am. Chem. Soc. 1963, 85, 2870–2871.
- 20
- 20aR. E. Rosenberg, R. L. Abel, M. D. Drake, D. J. Fox, A. K. Ignatz, D. M. Kwiat, K. M. Schaal, P. R. Virkler, J. Org. Chem. 2001, 66, 1694–1700;
- 20bJ. Graton, Z. Wang, A.-M. Brossard, D. Gonçalves Monteiro, J.-Y. Le Questel, B. Linclau, Angew. Chem. 2012, 124, 6280–6284;
10.1002/ange.201202059 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6176–6180.
- 21
- 21aG. Shaw, R. N. Warrener, J. Chem. Soc. 1958, 157–161;
- 21bA. N. Payne, S. M. Roberts, J. Chem. Soc. Perkin Trans. 1 1992, 2633–2641;
- 21cC. A. Fletcher, H. Hilpert, P. L. Myers, S. M. Roberts, R. Storer, J. Chem. Soc. Chem. Commun. 1989, 1707–1709.
- 22A. Stauffiger, C. J. Leumann, Eur. J. Org. Chem. 2009, 1153–1162.
- 23M. Y. Anzahaee, J. K. Watts, N. R. Alla, A. W. Nicholson, M. J. Damha, J. Am. Chem. Soc. 2011, 133, 728–731.
- 24
- 24aC. J. Wilds, M. J. Damha, Nucleic Acids Res. 2000, 28, 3625–3635;
- 24bI. Berger, V. Tereshki, H. Ikeda, V. E. Marquez, M. Egli, Nucleic Acids Res. 1998, 26, 2473–2480.