First Stabilization of 14-Electron Rhodium(I) Complexes by Hemichelation†
Christophe Werlé
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.fr
Search for more papers by this authorCorinne Bailly
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.fr
Search for more papers by this authorDr. Lydia Karmazin-Brelot
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.fr
Search for more papers by this authorXavier-Frédéric Le Goff
Département de Chimie, Ecole Polytechnique, Palaiseau cedex (France)
Search for more papers by this authorCorresponding Author
Dr. Michel Pfeffer
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.fr
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.frSearch for more papers by this authorCorresponding Author
Dr. Jean-Pierre Djukic
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.fr
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.frSearch for more papers by this authorChristophe Werlé
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.fr
Search for more papers by this authorCorinne Bailly
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.fr
Search for more papers by this authorDr. Lydia Karmazin-Brelot
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.fr
Search for more papers by this authorXavier-Frédéric Le Goff
Département de Chimie, Ecole Polytechnique, Palaiseau cedex (France)
Search for more papers by this authorCorresponding Author
Dr. Michel Pfeffer
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.fr
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.frSearch for more papers by this authorCorresponding Author
Dr. Jean-Pierre Djukic
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.fr
Institut de Chimie de Strasbourg, UMR 7177 CNRS, Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex (France) http://lcsom.u-strasbg.frSearch for more papers by this authorThis work was supported by the National Research Agency (ANR project WEAKINTERMET-2DA), the Laboratory of Excellence “Chemistry of Complex Systems” and the Centre National de la Recherche Scientifique.
Graphical Abstract
Abstract
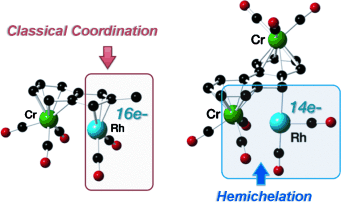
Hemichelation is emerging as a new mode of coordination where non-covalent interactions crucially contribute to the cohesion of electron-unsaturated organometallic complexes. This study discloses an unprecedented demonstration of this concept to a Group 9 metal, that is, RhI. The syntheses of new 14-electron RhI complexes were achieved by choosing the anti-[(η6:η6-fluorenyl){Cr(CO)3}2] anion as the ambiphilic hemichelating ligand, which was treated with [{Rh(nbd)Cl}2] (nbd=norbornadiene) and [{Rh(CO)2Cl}2]. The new T-shaped RhI hemichelates were characterized by analytical and structural methods. Investigations using the methods of the DFT and electron-density topology analysis (NCI region analysis, QTAIM theory) confirmed the closed-shell, non-covalent and attractive characters of the interaction between the RhI center and the proximal Cr(CO)3 moiety. This study shows that, by appropriate tuning of the electronic properties of the ambiphilic ligand, truly coordination-unsaturated RhI complexes can be synthesized in a manageable form.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201405240_sm_miscellaneous_information.pdf965.8 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. A. Casares, P. Espinet, G. Salas, Chem. Eur. J. 2002, 8, 4843–4853;
10.1002/1521-3765(20021104)8:21<4843::AID-CHEM4843>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 1bM. A. Ortuno, S. Conejero, A. Lledós, Beilstein J. Org. Chem. 2013, 9, 1352–1382;
- 1cY. W. Yared, S. L. Miles, R. Bau, C. A. Reed, J. Am. Chem. Soc. 1977, 99, 7076–7078;
- 1dJ. P. Stambuli, C. D. Incarvito, M. Bühl, J. F. Hartwig, J. Am. Chem. Soc. 2004, 126, 1184–1194;
- 1eJ. P. Stambuli, Z. Weng, C. D. Incarvito, J. F. Hartwig, Angew. Chem. 2007, 119, 7818–7821;
10.1002/ange.200702809 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7674–7677;
- 1fY. Feng, C. Wang, H. Fan, J. Organomet. Chem. 2011, 696, 4064–4069;
- 1gN. Chandrashekhar, V. Gayathri, N. M. N. Gowda, Polyhedron 2010, 29, 288–295;
- 1hA. Vigalok, Y. Ben-David, D. Milstein, Organometallics 1996, 15, 1839–1844;
- 1iG. Giordano, E. Rotondo, Polyhedron 1994, 13, 2507–2511;
- 1jY. Masashi, R. Noyori, Organometallics 1992, 11, 3167–3169;
- 1kA. A. H. Van der Zeijden, G. Van Koten, R. A. Nordemann, B. Kojic-Prodic, A. L. Spek, Organometallics 1988, 7, 1957–1966;
- 1lR. A. Gossage, H. A. Jenkins, N. D. Jones, R. C. Jones, B. F. Yates, Dalton Trans. 2008, 3115–3122;
- 1mD. V. Yandulov, N. T. Tran, J. Am. Chem. Soc. 2007, 129, 1342–1358;
- 1nV. P. Ananikov, D. G. Musaev, K. Morokuma, Eur. J. Inorg. Chem. 2007, 5390–5399;
- 1oC. Amatore, A. Bucaille, A. Fuxa, A. Jutand, G. Meyer, A. N. Ntepe, Chem. Eur. J. 2001, 7, 2134–2142;
10.1002/1521-3765(20010518)7:10<2134::AID-CHEM2134>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 1pK. Tatsumi, R. Hoffmann, A. Yamamoto, J. K. Stille, Bull. Chem. Soc. Jpn. 1981, 54, 1857–1867.
- 2
- 2aH. Urtel, C. Meier, F. Eisenträger, F. Rominger, J. P. Joschek, P. Hofmann, Angew. Chem. 2001, 113, 803–806;
10.1002/1521-3757(20010216)113:4<803::AID-ANGE8030>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 2001, 40, 781–784;10.1002/1521-3773(20010216)40:4<781::AID-ANIE7810>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 2bM. D. Walter, P. S. White, M. Brookhart, New J. Chem. 2013, 37, 1128–1133;
- 2cO. Rivada-Wheelaghan, M. A. Ortuno, J. Diez, A. Lledos, S. Conejero, Angew. Chem. 2012, 124, 4002–4005;
10.1002/ange.201200070 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3936–3939;
- 2dM. Hasegawa, Y. Segawa, M. Yamashita, K. Nozaki, Angew. Chem. 2012, 124, 7062–7066; Angew. Chem. Int. Ed. 2012, 51, 6956–6960.
- 3M. A. Ortuno, P. Vidossich, G. Ujaque, S. Conejero, A. Lledós, Dalton Trans. 2013, 42, 12165–12172.
- 4B. M. Emerich, C. E. Moore, B. J. Fox, A. L. Rheingold, J. S. Figueroa, Organometallics 2011, 30, 2598–2608.
- 5A. B. Chaplin, Organometallics 2014, 33, 624–626.
- 6
- 6aI. Hyla-Kryspin, S. Grimme, J.-P. Djukic, Organometallics 2009, 28, 1001–1013;
- 6bC. Werlé, X.-F. Le Goff, J.-P. Djukic, J. Organomet. Chem. 2014, 751, 754–759;
- 6cJ.-P. Djukic, C. Michon, A. Maisse-Francois, R. Allagapen, M. Pfeffer, K. H. Dotz, A. De Cian, J. Fischer, Chem. Eur. J. 2000, 6, 1064–1077;
10.1002/(SICI)1521-3765(20000317)6:6<1064::AID-CHEM1064>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 6dS. Büschel, A.-K. Jungton, T. Bannenberg, S. Randoll, C. G. Hirb, P. G. Jones, M. Tamm, Chem. Eur. J. 2009, 15, 2176–2184.
- 7
- 7aS. Grimme, J.-P. Djukic, Inorg. Chem. 2010, 49, 2911–2919;
- 7bS. Grimme, J.-P. Djukic, Inorg. Chem. 2011, 50, 2619–2628.
- 8
- 8aC. Werlé, C. Bailly, L. Karmazin-Brelot, X.-F. Le Goff, L. Ricard, J.-P. Djukic, J. Am. Chem. Soc. 2013, 135, 17839–17852;
- 8bC. Werlé, M. Hamdaoui, C. Bailly, X.-F. Le Goff, L. Brelot, J.-P. Djukic, J. Am. Chem. Soc. 2013, 135, 1715–1718.
- 9
- 9aS. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. 2011, 32, 1456–1465;
- 9bS. Grimme, R. Huenerbein, S. Ehrlich, ChemPhysChem 2011, 12, 1258–1261;
- 9cS. Grimme, Chem. Eur. J. 2012, 18, 9955–9964;
- 9dS. Grimme, M. Steinmetz, Phys. Chem. Chem. Phys. 2013, 15, 16031–16042;
- 9eT. Risthaus, S. Grimme, J. Chem. Theory Comput. 2013, 9, 1580–1591.
- 10G. Giordano, R. H. Crabtree, Inorg. Synth. 1990, 28, 88–90.
- 11E. W. Abel, M. A. Bennett, G. Wilkinson, J. Chem. Soc. A 1959, 3178–3182.
- 12D. Hanh Nguyen, N. Lassauque, L. Vendier, S. Mallet-Ladeira, C. Le Berre, P. Serp, P. Kalck, Eur. J. Inorg. Chem. 2014, 326–336.
- 13
- 13aA. Ceccon, A. Gambaro, S. Santi, G. Valle, A. Venzo, J. Chem. Soc. Chem. Commun. 1989, 51–53;
- 13bC. Bonifaci, A. Ceccon, A. Gambaro, P. Ganis, S. Santi, G. Valle, A. Venzo, Organometallics 1993, 12, 4211–4214;
- 13cA. Ceccon, C. J. Elsevier, J. M. Ernsting, A. Gambaro, S. Santi, A. Venzo, Inorg. Chim. Acta 1993, 204, 15–26;
- 13dC. Bonifaci, A. Ceccon, A. Gambaro, P. Ganis, S. Santi, A. Venzo, Organometallics 1995, 14, 2430–2434;
- 13eC. Bonifaci, A. Ceccon, S. Santi, C. Mealli, R. W. Zoellner, Inorg. Chim. Acta 1995, 240, 541–549;
- 13fC. Bonifaci, A. Ceccon, A. Gambaro, P. Ganis, S. Santi, G. Valle, A. Venzo, J. Organomet. Chem. 1995, 492, 35–39.
- 14T. Schwabe, S. Grimme, J.-P. Djukic, J. Am. Chem. Soc. 2009, 131, 14156–14157.
- 15F. Weinhold, C. R. Landis, Valency and Bonding, a natural bond orbital donor-acceptor perspective, University Press, Cambridge, 2005.
10.1017/CBO9780511614569 Google Scholar
- 16J. Tao, J. P. Perdew, V. N. Staroverov, G. E. Scuseria, Phys. Rev. Lett. 2003, 91, 146401.
- 17A. Ceccon, A. Gambaro, A. Venzo, V. Lucchini, T. E. Bitterwolf, J. Shade, J. Organomet. Chem. 1988, 349, 315–322.
- 18A. Bondi, J. Phys. Chem. 1964, 68, 441–451.
- 19
- 19aJ. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J. P. Piquemal, D. N. Beratan, W. Yang, J. Chem. Theory Comput. 2011, 7, 625–632;
- 19bE. Johnson, S. Keinan, P. Mori-Sanchez, J. Contreras-Garcia, A. J. Cohen, W. Yang, J. Am. Chem. Soc. 2010, 132, 6498–6506.
- 20
- 20aR. F. W. Bader, Atoms in Molecules: A Quantum Theory, Clarendon, Oxford, 1990;
10.1093/oso/9780198551683.001.0001 Google Scholar
- 20bP. Popelier, Atoms in Molecules, An Introduction, Prentice Hall, Harlow, England, 2000.
- 21K. B. Wiberg, Tetrahedron Lett. 1968, 24, 1083–1096.
- 22
- 22aM. P. Mitoraj, A. Michalak, T. Ziegler, Organometallics 2009, 28, 3727–3733;
- 22bM. P. Mitoraj, A. Michalak, T. Ziegler, J. Chem. Theory Comput. 2009, 5, 962–975.