N-Heterocyclic Carbene Coordinated Neutral and Cationic Heavier Cyclopropylidenes†
Dr. Anukul Jana
Krupp-Chair of General and Inorganic Chemistry, Saarland University, 66125 Saarbrücken (Germany) http://www.uni-saarland.de/fak8/scheschkewitz/index.html
Current address: Tata Institute of Fundamental Research, Centre for Interdisciplinary Sciences, 21, Brundavan Colony, Narsingi, Hyderabad-500075 (India)
Search for more papers by this authorDipl.-Chem. Isabell Omlor
Krupp-Chair of General and Inorganic Chemistry, Saarland University, 66125 Saarbrücken (Germany) http://www.uni-saarland.de/fak8/scheschkewitz/index.html
Search for more papers by this authorDr. Volker Huch
Krupp-Chair of General and Inorganic Chemistry, Saarland University, 66125 Saarbrücken (Germany) http://www.uni-saarland.de/fak8/scheschkewitz/index.html
Search for more papers by this authorProf. Dr. Henry S. Rzepa
Department of Chemistry, Imperial College London, London SW7 2AZ (UK)
Search for more papers by this authorCorresponding Author
Prof. Dr. David Scheschkewitz
Krupp-Chair of General and Inorganic Chemistry, Saarland University, 66125 Saarbrücken (Germany) http://www.uni-saarland.de/fak8/scheschkewitz/index.html
Krupp-Chair of General and Inorganic Chemistry, Saarland University, 66125 Saarbrücken (Germany) http://www.uni-saarland.de/fak8/scheschkewitz/index.htmlSearch for more papers by this authorDr. Anukul Jana
Krupp-Chair of General and Inorganic Chemistry, Saarland University, 66125 Saarbrücken (Germany) http://www.uni-saarland.de/fak8/scheschkewitz/index.html
Current address: Tata Institute of Fundamental Research, Centre for Interdisciplinary Sciences, 21, Brundavan Colony, Narsingi, Hyderabad-500075 (India)
Search for more papers by this authorDipl.-Chem. Isabell Omlor
Krupp-Chair of General and Inorganic Chemistry, Saarland University, 66125 Saarbrücken (Germany) http://www.uni-saarland.de/fak8/scheschkewitz/index.html
Search for more papers by this authorDr. Volker Huch
Krupp-Chair of General and Inorganic Chemistry, Saarland University, 66125 Saarbrücken (Germany) http://www.uni-saarland.de/fak8/scheschkewitz/index.html
Search for more papers by this authorProf. Dr. Henry S. Rzepa
Department of Chemistry, Imperial College London, London SW7 2AZ (UK)
Search for more papers by this authorCorresponding Author
Prof. Dr. David Scheschkewitz
Krupp-Chair of General and Inorganic Chemistry, Saarland University, 66125 Saarbrücken (Germany) http://www.uni-saarland.de/fak8/scheschkewitz/index.html
Krupp-Chair of General and Inorganic Chemistry, Saarland University, 66125 Saarbrücken (Germany) http://www.uni-saarland.de/fak8/scheschkewitz/index.htmlSearch for more papers by this authorSupport by the EPSRC (EP/H048804/1), the COST Action CM1302 (Smart Inorganic Polymers), and the Alfried Krupp von Bohlen und Halbach Foundation is gratefully acknowledged.
Graphical Abstract
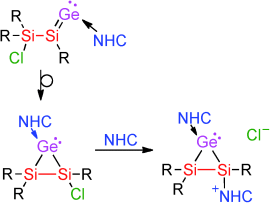
He is heavy and he′s my brother: NHC-coordinated cyclopropenylidene analogues with molecular scaffolds exclusively formed by heavier Group 14 elements are accessible from the corresponding vinylidene isomers by exchange of the NHC ligand for a smaller NHC. The residual chloride in one of these heavier cyclic carbenes can be expelled by a second equivalent of NHC to generate cationic derivatives of the imidazolium type.
Abstract
Cyclopropylidene is a transient intermediate of the allene–propyne–cyclopropene isomerization. The incorporation of heavier Group 14 elements into the cyclopropylidene scaffold has to date been restricted to the formal replacement of the carbenic carbon atom by a base-coordinated silicon(II) center. Herein we report the synthesis and characterization of NHC-coordinated heavier cyclopropylidenes (Si2GeR3X, and Si3R3Br; X=Cl, Mes; R=Tip=2,4,6-iPr3C6H2) in which the three-membered ring is exclusively formed by silicon and germanium. In case of the chloro-substituted Si2Ge-cyclopropylidene, a stable heavier cycloprop-1-yl-2-ylidene cation is obtained by NHC-induced chloride dissociation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201405238_sm_miscellaneous_information.pdf1.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Rubin, M. Rubina, V. Gevorgyan, Chem. Rev. 2007, 107, 3117–3179;
- 1bW. R. Dolbier, Jr., M. A. Battiste, Chem. Rev. 2003, 103, 1071–1098.
- 2
- 2aA. de Meijere, D. Faber, M. Noltemeyer, R. Boese, T. Haumann, T. Müller, M. Bendikov, E. Matzner, Y. Apeloig, J. Org. Chem. 1996, 61, 8564–8568;
- 2bK. Komatsu, T. Kitagawa, Chem. Rev. 2003, 103, 1371–1427;
- 2cI. Fernández, M. Duvall, J. I.-C. Wu, P. von R. Schleyer, G. Frenking, Chem. Eur. J. 2011, 17, 2215–2224.
- 3Germanium:
- 3aA. Sekiguchi, H. Yamazaki, C. Kabuto, H. Sakurai, J. Am. Chem. Soc. 1995, 117, 8025–8026;
- 3bA. Sekiguchi, M. Tsukamoto, M. Ichinohe, Science 1997, 275, 60–61; Silicon:
- 3cT. Iwamoto, C. Kabuto, M. Kira, J. Am. Chem. Soc. 1999, 121, 886–887;
- 3dM. Ichinohe, T. Matsuno, A. Sekiguchi, Angew. Chem. 1999, 111, 2331–2333;
10.1002/(SICI)1521-3757(19990802)111:15<2331::AID-ANGE2331>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2194–2196;10.1002/(SICI)1521-3773(19990802)38:15<2194::AID-ANIE2194>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 3eA. Sekiguchi, V. Y. Lee, Chem. Rev. 2003, 103, 1429–1447;
- 3fM. Ichinohe, M. Igarashi, K. Sanuki, A. Sekiguchi, J. Am. Chem. Soc. 2005, 127, 9978–9979;
- 3gV. Y. Lee, A. Sekiguchi, Acc. Chem. Res. 2007, 40, 410–419; Tin:
- 3hN. Wiberg, H.-W. Lerner, S. K. Vasisht, S. Wagner, K. Karaghiosoff, H. Nöth, W. Ponikwar, Eur. J. Inorg. Chem. 1999, 1211–1218.
10.1002/(SICI)1099-0682(199908)1999:8<1211::AID-EJIC1211>3.0.CO;2-0 CAS Web of Science® Google Scholar
- 4The related cyclopropenylidenes have been isolated as stable amino-substituted derivatives:
- 4aV. Lavallo, Y. Canac, B. Donnadieu, W. W. Schoeller, G. Bertrand, Science 2006, 312, 722–724;
- 4bV. Lavallo, Y. Ishida, B. Donnadieu, G. Bertrand, Angew. Chem. 2006, 118, 6804–6807;
10.1002/ange.200602701 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6652–6655.
- 5
- 5aW. von E. Doering, P. M. LaFlamme, Tetrahedron 1958, 14, 75–79;
10.1016/0040-4020(58)88025-4 Google Scholar
- 5bA. Rauk, W. J. Bouma, L. Radom, J. Am. Chem. Soc. 1985, 107, 3780–3786;
- 5cP. Valtazanos, K. Ruedenberg, Theor. Chim. Acta 1991, 78, 397–416;
- 5dM. S. Baird, C. M. Dale, J. R. Al Dulayymi, J. Chem. Soc. Perkin Trans. 1 1993, 1373–1374;
- 5eH. F. Bettinger, P. R. Schreiner, P. v. R. Schleyer, H. F. Schaefer III, J. Phys. Chem. 1996, 100, 16147–16154;
- 5fA. de Meijere, D. Faber, U. Heinecke, R. Walsh, T. Müller, Y. Apeloig, Eur. J. Org. Chem. 2001, 663–680;
- 5gL. K. Sydnes, Chem. Rev. 2003, 103, 1133–1150.
- 6
- 6aM. Kosa, M. Karni, Y. Apeloig, J. Am. Chem. Soc. 2004, 126, 10544–10545;
- 6bM. Kosa, M. Karni, Y. Apeloig, J. Chem. Theory Comput. 2006, 2, 956–964;
- 6cB. Pintér, A. Olasz, K. Petrov, T. Veszprémi, Organometallics 2007, 26, 3677–3683.
- 7S. Ishida, T. Iwamoto, C. Kabuto, M. Kira, Nature 2003, 421, 725–727.
- 8aA. Sekiguchi, R. Kinjo, M. Ichinohe, Science 2004, 305, 1755–1757;
- 8bN. Wiberg, S. K. Vasisht, G. Fischer, P. Mayer, Z. Anorg. Allg. Chem. 2004, 630, 1823–1828.
- 9
- 9aD. Martin, M. Soleilhavoup, G. Bertrand, Chem. Sci. 2011, 2, 389–399;
- 9bY. Wang, G. H. Robinson, Inorg. Chem. 2011, 50, 12326–12337;
- 9cY. Xiong, S. Yao, M. Driess, Angew. Chem. 2013, 125, 4398–4407; Angew. Chem. Int. Ed. 2013, 52, 4302–4311;
- 9dR. S. Ghadwal, R. Azhakar, H. W. Roesky, Acc. Chem. Res. 2013, 46, 444–456;
- 9eC. D. Martin, M. Soleilhavoup, G. Bertrand, Chem. Sci. 2013, 4, 3020–3030.
- 10K. Leszczyńska, K. Abersfelder, A. Mix, B. Neumann, H.-G. Stammler, M. J. Cowley, P. Jutzi, D. Scheschkewitz, Angew. Chem. 2012, 124, 6891–6895;
10.1002/ange.201202277 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6785–6788.
- 11M. J. Cowley, V. Huch, H. S. Rzepa, D. Scheschkewitz, Nat. Chem. 2013, 5, 876–879.
- 12A. Jana, M. Majumder, V. Huch, M. Zimmer, D. Scheschkewitz, Dalton Trans. 2014, 43, 5175–5181.
- 13A. Jana, V. Huch, D. Scheschkewitz, Angew. Chem. 2013, 125, 12401–12404; Angew. Chem. Int. Ed. 2013, 52, 12179–12182.
- 14M. Y. Abraham, Y. Wang, Y. Xie, P. Wei, H. F. Schaefer III, P. v. R. Schleyer, G. H. Robinson, J. Am. Chem. Soc. 2011, 133, 8874–8876.
- 15R. Rodriguez, T. Troadec, T. Kato, N. S. Merceron, J.-M. Sotiropoulos, A. Baceiredo, Angew. Chem. 2012, 124, 7270–7273; Angew. Chem. Int. Ed. 2012, 51, 7158–7161.
- 16
- 16aD. Himmel, I. Krossing, A. Schnepf, Angew. Chem. 2014, 126, 378–382;
10.1002/ange.201300461 Google ScholarAngew. Chem. Int. Ed. 2014, 53, 370–374;
- 16bG. Frenking, Angew. Chem. 2014, 126, 6152–6158; Angew. Chem. Int. Ed. 2014, 53, 6040–6046.
- 17A. C. Filippou, Y. N. Lebedev, O. Chernov, M. Straßmann, G. Schnakenburg, Angew. Chem. 2013, 125, 7112–7116;
10.1002/ange.201301363 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 6974–6978.
- 18T. Yamaguchi, A. Sekiguchi, M. Driess, J. Am. Chem. Soc. 2010, 132, 14061–14063.
- 19Experimental details are supplied in the Supporting Information. Computational details and interactive 3D models are available via the Interactivity Box, DOI: and the further digital repository DOIs therein. CCDC 977842 (5), 967887 (6), 967888 (7), 967889 (12), 967890 (13), contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 20N. Kuhn, T. Kratz, Synthesis 1993, 561–562.
- 21A. C. Filippou, O. Chernov, B. Blom, K. W. Stumpf, G. Schnakenburg, Chem. Eur. J. 2010, 16, 2866–2872.
- 22
- 22aM. Ichinohe, K. Sanuki, S. Inoue, A. Sekiguchi, Organometallics 2004, 23, 3088–3090;
- 22bV. Y. Lee, A. Sekiguchi, Chem. Lett. 2004, 33, 84–85.
- 23V. Y. Lee, M. Ichinohe, A. Sekiguchi, J. Am. Chem. Soc. 2000, 122, 9034–9035.
- 24
- 24aM. Weidenbruch, S. Willms, W. Saak, G. Henkel, Angew. Chem. 1997, 109, 2612–2613;
10.1002/ange.19971092231 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 2503–2504;
- 24bD. Scheschkewitz, Angew. Chem. 2004, 116, 3025–3028; Angew. Chem. Int. Ed. 2004, 43, 2965–2967.
- 25
- 25aD. Scheschkewitz, Angew. Chem. 2005, 117, 3014–3016;
10.1002/ange.200462730 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2954–2956;
- 25bK. Abersfelder, D. Scheschkewitz, J. Am. Chem. Soc. 2008, 130, 4114–4121;
- 25cK. Abersfelder, A. J. P. White, H. S. Rzepa, D. Scheschkewitz, Science 2010, 327, 564–566.
- 26See Supporting Information for the solid state structure of compound 13.