Adsorption of I2 by Macrocyclic Polyazadithiophenolato Complexes Mediated by Charge-Transfer Interactions†
Matthias Golecki
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)
Search for more papers by this authorNorman Beyer
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)
Search for more papers by this authorDr. Gunther Steinfeld
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)
Search for more papers by this authorDr. Vasile Lozan
Laboratory of Coordination Chemistry, Institute of Chemistry, Academy of Sciences of Moldova, Chisinau (Republic Moldova)
Search for more papers by this authorDr. Sergei Voitekhovich
Research Institute for Physical Chemical Problems, Belarusian State University, Leningradskaya 14, 220030 Minsk (Belarus)
Search for more papers by this authorDr. Muhamed Sehabi
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)
Search for more papers by this authorDr. Jens Möllmer
Institut für Nichtklassische Chemie e.V., Leipzig (Germany)
Search for more papers by this authorProf. Dr. Hans-Jörg Krüger
Fachbereich Chemie, TU Kaiserslautern (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Berthold Kersting
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)Search for more papers by this authorMatthias Golecki
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)
Search for more papers by this authorNorman Beyer
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)
Search for more papers by this authorDr. Gunther Steinfeld
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)
Search for more papers by this authorDr. Vasile Lozan
Laboratory of Coordination Chemistry, Institute of Chemistry, Academy of Sciences of Moldova, Chisinau (Republic Moldova)
Search for more papers by this authorDr. Sergei Voitekhovich
Research Institute for Physical Chemical Problems, Belarusian State University, Leningradskaya 14, 220030 Minsk (Belarus)
Search for more papers by this authorDr. Muhamed Sehabi
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)
Search for more papers by this authorDr. Jens Möllmer
Institut für Nichtklassische Chemie e.V., Leipzig (Germany)
Search for more papers by this authorProf. Dr. Hans-Jörg Krüger
Fachbereich Chemie, TU Kaiserslautern (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Berthold Kersting
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)
Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig (Germany)Search for more papers by this authorWe thank the Deutsche Forschungsgemeinschaft (Project KE 585/8-1) and the Universität Leipzig for funding. We are also grateful to Prof. Dr. H. Krautscheid for providing facilities for X-ray crystallographic measurements.
Graphical Abstract
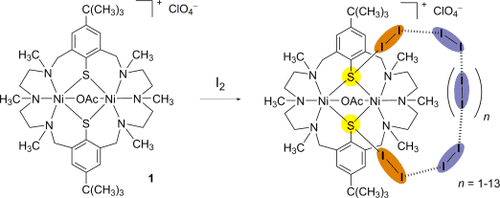
Seeing I to I: The macrocyclic complex [Ni2(L)(OAc)]ClO4 (1) adsorbs up to 17 molar equivalents (>270 wt %) of iodine, although it does not exhibit permanent porosity. IR and crystallographic studies reveal that two I2 molecules are captured by means of thiophenolate→I2 charge-transfer interactions, which enable the diffusion and sorption of further I2 molecules in a polyiodide-like network.
Abstract
The macrocyclic complex [Ni2(L)(OAc)]ClO4 (1) adsorbs up to 17 molar equivalents (>270 wt %) of iodine, although it does not exhibit permanent porosity. Vibrational spectroscopic and crystallographic studies reveal that two I2 molecules are captured by means of thiophenolate→I2 charge-transfer interactions, which enable the diffusion and sorption of further I2 molecules in a polyiodide-like network. The efficient sorption and desorption characteristics make this material suitable for accommodation, sensing, and slow release of I2.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201405199_sm_miscellaneous_information.pdf417.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. L. Schmidt, P. M. Skarstad, J. Power Sources 1997, 65, 121–128.
- 2K. Reimer, P. M. Vogt, B. Broegmann, J. Hauser, O. Rossbach, A. Kramer, P. Rudolph, B. Bosse, H. Schreier, W. Fleischer, Dermatology 2000, 201, 235–241.
- 3
- 3aB. O’Regan, M. Grätzel, Nature 1991, 353, 737–740;
- 3bG. Boschloo, A. Hagfeldt, Acc. Chem. Res. 2009, 42, 1819–1826;
- 3cG. Lota, K. Fic, E. Frackowiak, Electrochem. Commun. 2011, 13, 38–41.
- 4The chemistry of polyiodides has been thoroughly investigated, see:
- 4aK. F. Tebbe, R. Buchem, Angew. Chem. 1997, 109, 1403–1405;
10.1002/ange.19971091233 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1345–1346;
- 4bI. Pantenburg, I. Mueller, K. F. Tebbe, Z. Anorg. Allg. Chem. 2005, 631, 654–658.
- 5P. H. Svensson, L. Kloo, Chem. Rev. 2003, 103, 1649–1684.
- 6M. Wolff, J. Meyer, C. Feldmann, Angew. Chem. 2011, 123, 5073–5077; Angew. Chem. Int. Ed. 2011, 50, 4970–4973.
- 7H. Haller, M. Ellwanger, A. Higelin, S. Riedel, Angew. Chem. 2011, 123, 11732–11736;
10.1002/ange.201105237 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11528–11532.
- 8Halogen adducts of regular electron-pair donors (alcohols, ethers, thioethers, thiones) are quite common:
- 8aA. J. Blake, F. A. Devillanova, R. O. Gould, W.-S. Li, V. Lippolis, S. Parsons, C. Radek, M. Schröder, Chem. Soc. Rev. 1998, 27, 195–205;
- 8bH. Bock, Z. Havlas, A. Rauschenbach, C. Näther, M. Kleine, Chem. Commun. 1996, 1529–1531;
- 8cM. C. Aragoni, M. Arca, F. Demartin, F. A. Devillanova, A. Garau, F. Isaia, F. Lelj, V. Lippolis, G. Verani, Chem. Eur. J. 2001, 7, 3122–3133.
10.1002/1521-3765(20010716)7:14<3122::AID-CHEM3122>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 9E. J. Lyon, G. Musie, J. H. Reibenspies, M. Y. Darensbourg, Inorg. Chem. 1998, 37, 6942–6946.
- 10G. Steinfeld, V. Lozan, H.-J. Krüger, B. Kersting, Angew. Chem. 2009, 121, 1988–1991;
10.1002/ange.200805028 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 1954–1957.
- 11G. Wirnsberger, H. P. Fritzer, A. Popitsch, G. van der Goor, P. Behrens, Angew. Chem. 1996, 108, 2951–2953;
10.1002/ange.19961082310 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 2777–2779.
- 12T. Hertzsch, F. Budde, E. Weber, J. Hulliger, Angew. Chem. 2002, 114, 2385–2388;
10.1002/1521-3757(20020703)114:13<2385::AID-ANGE2385>3.0.CO;2-Y Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2281–2284.
- 13
- 13aH. J. Choi, M. P. Suh, J. Am. Chem. Soc. 2004, 126, 15844–15851;
- 13bM.-H. Zeng, Q.-X. Wang, Y.-X. Tan, S. Hu, H.-X. Zhao, L.-S. Long, M. Kurmoo, J. Am. Chem. Soc. 2010, 132, 2561–2563.
- 14F. A. Cotton, E. V. Dikarev, M. A. Petrukhina, Angew. Chem. 2000, 112, 2452–2454;
10.1002/1521-3757(20000703)112:13<2452::AID-ANGE2452>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2362–2364.10.1002/1521-3773(20000703)39:13<2362::AID-ANIE2362>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 15Y. Krupskaya, A. Alfonsov, A. Parameswaran, V. Kataev, R. Klingeler, G. Steinfeld, N. Beyer, M. Gressenbuch, B. Kersting, B. Büchner, ChemPhysChem 2010, 11, 1961–1970.
- 16N. Herzmann, I. Pantenburg, I. Müller, W. Tyrra, G. Meyer, Z. Anorg. Allg. Chem. 2006, 632, 2209–2216.
- 17I. L. Karle, J. Chem. Phys. 1955, 23, 1739–1740.
- 18R. Minkwitz, H. Preut, J. Sawatzki, Z. Naturforsch. B 1988, 43, 399–402.
- 19T. Klapötke, J. Passmore, Acc. Chem. Res. 1989, 22, 234–240.
- 20J. Allshouse, R. C. Haltiwanger, V. Allured, M. R. DuBois, Inorg. Chem. 1994, 33, 2505–2506.
- 21Crystal size <0.1 μm (estimated by optical microscopy).
- 22Y. Wang, G. A. Sotzing, R. A. Weiss, Polymer 2006, 47, 2728–2740.
- 23C. R. Fox, “Industrial wastewater control and recovery of organic chemicals by adsorption” in Adsorption Technology, a Step by Step Approach to Process Evaluation and Application, Chemical Industries Series, Vol. 19 (Ed.: F. L. Slejko), Marcel Dekker, New York, 1985, pp. 167–182.
- 24Under our experimental conditions, the iodine adsorption capacity of MgSO4, CaCl2, and Ni(OAc)2⋅4 H2O are 17, 300, and 87 mg g−1 (at 298 K, pi(I2)=45 Pa, 1600 h).
- 25C. E. Rogers in Polymer Permeability (Ed.: ), Elsevier Applied Science, London, 1986, Chap. 2.
- 26 Diffusion in Polymers (Eds.: ), Academic Press, London, 1968.
- 27One can roughly estimate the diffusion constant D [cm2 s−1]: If L is taken as the distance from the surface to the center of the microcrystalline sample (∼0.2 cm), then D (21 °C)=0.00027 (h−1)×(0.2 cm)2/3600 (s h−1)=3×10−9 cm2 s−1; D(60 °C)=0.0019 (h−1)×(0.2 cm)2/3600 (s h−1)=2×10−8 cm2 s−1. However, these values should considered to be indicative rather than definitive.
- 28I2 sorption has been observed for other metal complexes supported by the N6S2 macrocycle [M2L(μ-L′)]ClO4 (e.g. M=NiII, L′=O2COMe, nsat294 K=10.3; M=ZnII, L′=OAc, nsat=15.0; M=CoII, L′=OAc, nsat=7.2). It is concluded that it is a general property of thiolato-bridged metal complexes.
- 29A comparison of the infrared spectra of [Ni2L(OAc)](ClO4) (1) and [Ni2L(OAc)(I2)2](I2)x(ClO4) (sample III) shows small shifts of the (triply spit) ν3 and ν4 vibrations of the ClO4− ion (Figure SI-4). This suggests that the iodine also interacts with the ClO4− ions. The Raman spectrum (Figure SI-5) of sample III shows strong peaks at 170, 231, and 233 cm−1 which are attributed to the II and RSI2 stretching vibrations. W. Kiefer, H. Herbstein, J. Raman Spectrosc. 1973, 1, 417–431.