One-Pot Synthesis of Highly Substituted Polyheteroaromatic Compounds by Rhodium(III)-Catalyzed Multiple CH Activation and Annulation†
Jayachandran Jayakumar
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorDr. Kanniyappan Parthasarathy
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorYi-Hsiang Chen
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorTai-Hua Lee
Department of Applied Chemistry, National Chiao Tung University, Hsinchu 30013 (Taiwan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shih-Ching Chuang
Department of Applied Chemistry, National Chiao Tung University, Hsinchu 30013 (Taiwan)
Shih-Ching Chuang, Department of Applied Chemistry, National Chiao Tung University, Hsinchu 30013 (Taiwan)
Chien-Hong Cheng, Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorCorresponding Author
Prof. Dr. Chien-Hong Cheng
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Shih-Ching Chuang, Department of Applied Chemistry, National Chiao Tung University, Hsinchu 30013 (Taiwan)
Chien-Hong Cheng, Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorJayachandran Jayakumar
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorDr. Kanniyappan Parthasarathy
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorYi-Hsiang Chen
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorTai-Hua Lee
Department of Applied Chemistry, National Chiao Tung University, Hsinchu 30013 (Taiwan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shih-Ching Chuang
Department of Applied Chemistry, National Chiao Tung University, Hsinchu 30013 (Taiwan)
Shih-Ching Chuang, Department of Applied Chemistry, National Chiao Tung University, Hsinchu 30013 (Taiwan)
Chien-Hong Cheng, Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorCorresponding Author
Prof. Dr. Chien-Hong Cheng
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Shih-Ching Chuang, Department of Applied Chemistry, National Chiao Tung University, Hsinchu 30013 (Taiwan)
Chien-Hong Cheng, Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorWe thank the Ministry of Science and Technology of the Republic of China (NSC 102-2628-M-007-005) for support of this research.
Graphical Abstract
Abstract
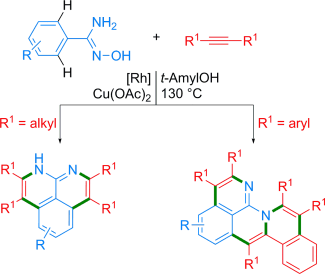
A new method for the synthesis of highly substituted naphthyridine-based polyheteroaromatic compounds in high yields proceeds through rhodium(III)-catalyzed multiple CH bond cleavage and CC and CN bond formation in a one-pot process. Such highly substituted polyheteroaromatic compounds have attracted much attention because of their unique π-conjugation, which make them suitable materials for organic semiconductors and luminescent materials. Furthermore, a possible mechanism, which involves multiple chelation-assisted ortho CH activation, alkyne insertion, and reductive elimination, is proposed for this transformation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201405183_sm_miscellaneous_information.pdf4.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Catellani, Top. Organomet. Chem. 2005, 8, 21;
10.1007/b104126 Google Scholar
- 1bM. Catellani, E. Motti, N. D. Ca, Acc. Chem. Res. 2008, 41, 1512;
- 1cC.-H. Jun, C. W. Moon, D.-Y. Lee, Chem. Eur. J. 2002, 8, 2422;
10.1002/1521-3765(20020603)8:11<2422::AID-CHEM2422>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 1dY. J. Park, C.-H. Jun, Bull. Korean Chem. Soc. 2005, 26, 877;
- 1eL. Ackermann, R. Vicente, Top. Curr. Chem. 2009, 292, 211;
- 1fK. Fagnou, Top. Curr. Chem. 2009, 292, 35;
- 1gX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196; Angew. Chem. Int. Ed. 2009, 48, 5094;
- 1hD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 1iT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147.
- 2For selected examples of rhodium-catalyzed CH activation and annulation with alkynes, see:
- 2aK. Ueura, T. Satoh, M. Miura, Org. Lett. 2007, 9, 1407;
- 2bK. Ueura, T. Satoh, M. Miura, J. Org. Chem. 2007, 72, 5362;
- 2cD. R. Stuart, M. Bertrand-Laperle, K. M. N. Burgess, K. Fagnou, J. Am. Chem. Soc. 2008, 130, 16474;
- 2dS. Mochida, K. Hirano, T. Satoh, M. Miura, J. Org. Chem. 2009, 74, 6295;
- 2eT. Fukutani, N. Umeda, K. Hirano, T. Satoh, M. Miura, Chem. Commun. 2009, 5141;
- 2fP. Too, Y. F. Wang, S. Chiba, Org. Lett. 2010, 12, 5688;
- 2gY. Su, M. Zhao, K. Han, G. Song, X. Li, Org. Lett. 2010, 12, 5462;
- 2hJ. Chen, G. Song, C. Pan, X. Li, Org. Lett. 2010, 12, 5426;
- 2iK. Morimoto, K. Hirano, T. Satoh, M. Miura, Org. Lett. 2010, 12, 2068;
- 2jD. R. Stuart, P. Alsabeh, M. Kuhn, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 18326;
- 2kS. Rakshit, F. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9585;
- 2lN. Guimond, C. Gouliaras, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 6908;
- 2mS. Mochida, M. Shimizu, K. Hirano, T. Satoh, M. Miura, Chem. Asian J. 2010, 5, 847;
- 2nX. Wei, M. Zhao, Z. Du, X. Li, Org. Lett. 2011, 13, 4636;
- 2oP. C. Too, S. H. Chua, S. H. Wong, S. Chiba, J. Org. Chem. 2011, 76, 6159;
- 2pN. Umeda, K. Hirano, T. Satoh, N. Shibata, H. Sato, M. Miura, J. Org. Chem. 2011, 76, 13;
- 2qX. Zhang, D. Chen, M. Zhao, J. Zhao, A. Jia, X. Li, Adv. Synth. Catal. 2011, 353, 719;
- 2rL. Zheng, J. Ju, Y. Bin, R. Hua, J. Org. Chem. 2012, 77, 5794;
- 2sW. Dong, L. Wang, K. Parthasarathy, F. F. Pan, C. Bolm, Angew. Chem. 2013, 125, 11787; Angew. Chem. Int. Ed. 2013, 52, 11573;
- 2tM. V. Pham, N. Cramer, Angew. Chem. 2014, 126, 3552; Angew. Chem. Int. Ed. 2014, 53, 3484.
- 3
- 3aL. Ackermann, A. V. Lygin, N. Hofmann, Angew. Chem. 2011, 123, 6503; Angew. Chem. Int. Ed. 2011, 50, 6379;
- 3bB. Li, H. Feng, S. Xu, B. Wang, Chem. Eur. J. 2011, 17, 12573;
- 3cL. Ackermann, A. V. Lygin, N. Hofmann, Org. Lett. 2011, 13, 3278;
- 3dL. Ackermann, S. Fenner, Org. Lett. 2011, 13, 6548;
- 3eR. K. Chinnagolla, M. Jeganmohan, Chem. Commun. 2012, 48, 2030;
- 3fL. Ackermann, L. Wang, A. V. Lygin, Chem. Sci. 2012, 3, 177;
- 3gK. R. Chinnagolla, M. Jeganmohan, Eur. J. Org. Chem. 2012, 417;
- 3hL. Ackermann, A. V. Lygin, Org. Lett. 2012, 14, 764;
- 3iL. Ackermann, J. Pospech, K. Graczyk, K. Rauch, Org. Lett. 2012, 14, 930;
- 3jR. K. Chinnagolla, S. Pimparkar, M. Jeganmohan, Org. Lett. 2012, 14, 3032;
- 3kV. S. Thirunavukkarasu, M. Donati, L. Ackermann, Org. Lett. 2012, 14, 3416.
- 4
- 4aT. Satoh, M. Miura, Chem. Eur. J. 2010, 16, 11212;
- 4bJ. Wencel-Delord, T. Dröge, F. Liu, F. Glorius, Chem. Soc. Rev. 2011, 40, 4740;
- 4cG. Song, F. Wang, X. Li, Chem. Soc. Rev. 2012, 41, 3651;
- 4dF. W. Patureau, J. Wencel-Delord, F. Glorius, Aldrichimica Acta 2012, 45, 31;
- 4eL. Ackermann, Acc. Chem. Res. 2014, 47, 281;
- 4fP. B. Arockiam, C. Bruneau, P. H. Dixneuf, Chem. Rev. 2012, 112, 5879;
- 4gJ. Li, M. John, L. Ackermann, Chem. Eur. J. 2014, 20, 5403.
- 5aN. Umeda, H. Tsurugi, T. Satoh, M. Miura, Angew. Chem. 2008, 120, 4083;
10.1002/ange.200800924 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4019;
- 5bS. Mochida, N. Umeda, K. Hirano, T. Satoh, M. Miura, Chem. Lett. 2010, 39, 744;
- 5cT. Fukutani, K. Hirano, T. Satoh, M. Miura, J. Org. Chem. 2011, 76, 2867.
- 6
- 6aG. Song, D. Chen, C. Pan, R. H. Crabtree, X. Li, J. Org. Chem. 2010, 75, 7487;
- 6bG. Song, X. Gong, X. Li, J. Org. Chem. 2011, 76, 7583.
- 7J. Huang, L. Dong, B. Han, C. Peng, Y. Chen, Chem. Eur. J. 2012, 18, 8896.
- 8X. Tan, B. Liu, X. Li, B. Li, S. Xu, H. Song, B. Wang, J. Am. Chem. Soc. 2012, 134, 16163.
- 9
- 9aV. S. Thirunavukkarasu, K. Parthasarathy, C.-H. Cheng, Angew. Chem. 2008, 120, 9604;
10.1002/ange.200804153 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9462;
- 9bV. S. Thirunavukkarasu, K. Parthasarathy, C.-H. Cheng, Chem. Eur. J. 2010, 16, 1436;
- 9cP. Gandeepan, K. Parthasarathy, C.-H. Cheng, J. Am. Chem. Soc. 2010, 132, 8569;
- 9dJ. Karthikeyan, C.-H. Cheng, Angew. Chem. 2011, 123, 10054;
10.1002/ange.201104311 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 9880;
- 9eJ. Karthikeyan, R. Haridharan, C.-H. Cheng, Angew. Chem. 2012, 124, 12509;
10.1002/ange.201206890 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12343;
- 9fK. Parthasarathy, M. Jeganmohan, C.-H. Cheng, Org. Lett. 2008, 10, 325;
- 9gK. Parthasarathy, C.-H. Cheng, J. Org. Chem. 2009, 74, 9359;
- 9hJ. Jayakumar, K. Parthasarathy, C.-H. Cheng, Angew. Chem. 2012, 124, 201;
10.1002/ange.201105755 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 197.
- 10C. White, A. Yates, P. M. Maitlis, Inorg. Synth. 1992, 29, 228.
- 11
- 11aJ. E. Anthony, Angew. Chem. 2008, 120, 460;
10.1002/ange.200604045 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 452;
- 11bM. D. Watson, A. Fechtenkötter, K. Müllen, Chem. Rev. 2001, 101, 1267;
- 11cU. Mitschke, P. Bäuerle, J. Mater. Chem. 2000, 10, 1471;
- 11dR. G. Harvey, Polycyclic aromatic hydrocarbons, Wiley-VCH, Weinheim, 1996;
- 11eM. Kimura, K. Tanaka, Angew. Chem. 2008, 120, 9914;
10.1002/ange.200804404 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9768.
- 12
- 12aB. Clement, M. Weide, U. Wolschendorf, I. Kock, Angew. Chem. 2005, 117, 641;
10.1002/ange.200461969 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 635;
- 12bC. Bailly, Chem. Rev. 2012, 112, 3611;
- 12cJ. Deguchi, T. Shoji, A. E. Nugroho, Y. Hirasawa, T. Hosoya, O. Shirota, K. Awang, A. H. A. Hadi, H. Morita, J. Nat. Prod. 2010, 73, 1727.
- 13See the Supporting Information. CCDC 997429 (1 b), CCDC 997432 (3 c), CCDC 997433 (3 i), CCDC 997430 (3 q), CCDC 997431 (3 s), and CCDC 998572 (3 t) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.