A High-Pressure NMR Probe for Aqueous Geochemistry†
Dr. Brent G. Pautler
Department of Chemistry, University of California, Davis, 1 Shields Ave, Davis, CA 95616 (USA)
Search for more papers by this authorChristopher A. Colla
Department of Earth and Planetary Sciences, University of California, Davis (USA)
Search for more papers by this authorDr. Rene L. Johnson
Department of Chemistry, University of California, Davis, 1 Shields Ave, Davis, CA 95616 (USA)
Search for more papers by this authorPeter Klavins
Department of Physics, University of California, Davis (USA)
Search for more papers by this authorDr. Stephen J. Harley
Energetic Materials Division, Lawrence Livermore National Laboratory, 7000 East Ave. Livermore, CA 94550 (USA)
Search for more papers by this authorDr. C. André Ohlin
School of Chemistry, Monash University, Wellington Rd, Clayton VIC 3800 (Australia)
Search for more papers by this authorProf. Dimitri A. Sverjensky
Department of Earth & Planetary Sciences, Johns Hopkins University, Baltimore, MD 21218 (USA)
Geophysical Laboratory, Carnegie Institution of Washington, Washington, DC 20015 (USA)
Search for more papers by this authorDr. Jeffrey H. Walton
NMR Facility, University of California, Davis (USA)
Search for more papers by this authorCorresponding Author
Prof. William H. Casey
Department of Chemistry, University of California, Davis, 1 Shields Ave, Davis, CA 95616 (USA)
Department of Earth and Planetary Sciences, University of California, Davis (USA)
Department of Chemistry, University of California, Davis, 1 Shields Ave, Davis, CA 95616 (USA)Search for more papers by this authorDr. Brent G. Pautler
Department of Chemistry, University of California, Davis, 1 Shields Ave, Davis, CA 95616 (USA)
Search for more papers by this authorChristopher A. Colla
Department of Earth and Planetary Sciences, University of California, Davis (USA)
Search for more papers by this authorDr. Rene L. Johnson
Department of Chemistry, University of California, Davis, 1 Shields Ave, Davis, CA 95616 (USA)
Search for more papers by this authorPeter Klavins
Department of Physics, University of California, Davis (USA)
Search for more papers by this authorDr. Stephen J. Harley
Energetic Materials Division, Lawrence Livermore National Laboratory, 7000 East Ave. Livermore, CA 94550 (USA)
Search for more papers by this authorDr. C. André Ohlin
School of Chemistry, Monash University, Wellington Rd, Clayton VIC 3800 (Australia)
Search for more papers by this authorProf. Dimitri A. Sverjensky
Department of Earth & Planetary Sciences, Johns Hopkins University, Baltimore, MD 21218 (USA)
Geophysical Laboratory, Carnegie Institution of Washington, Washington, DC 20015 (USA)
Search for more papers by this authorDr. Jeffrey H. Walton
NMR Facility, University of California, Davis (USA)
Search for more papers by this authorCorresponding Author
Prof. William H. Casey
Department of Chemistry, University of California, Davis, 1 Shields Ave, Davis, CA 95616 (USA)
Department of Earth and Planetary Sciences, University of California, Davis (USA)
Department of Chemistry, University of California, Davis, 1 Shields Ave, Davis, CA 95616 (USA)Search for more papers by this authorThis work is supported by the Department of Energy grant DE-FG02-05ER15693 and by LLNL under Contract DE-AC52-07NA2734 LLNL-JRNL-654755. Additional funding sources and awknowledgements are listed in the Supporting Information.
Graphical Abstract
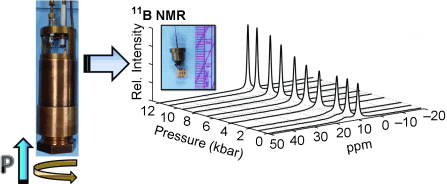
A non-magnetic piston-cylinder pressure cell has been developed for solution-state NMR spectroscopy up to 20 kbar for aqueous geochemical applications. 11B NMR spectroscopic investigations into the H3BO3–catechol equilibrium demonstrates a large pressure-driven exchange rate. The success of these experiments suggests that this probe design can be applied to a wide variety of NMR-active nuclei.
Abstract
A non-magnetic piston-cylinder pressure cell is presented for solution-state NMR spectroscopy at geochemical pressures. The probe has been calibrated up to 20 kbar using in situ ruby fluorescence and allows for the measurement of pressure dependencies of a wide variety of NMR-active nuclei with as little as 10 μL of sample in a microcoil. Initial 11B NMR spectroscopy of the H3BO3–catechol equilibria reveals a large pressure-driven exchange rate and a negative pressure-dependent activation volume, reflecting increased solvation and electrostriction upon boron-catecholate formation. The inexpensive probe design doubles the current pressure range available for solution NMR spectroscopy and is particularly important to advance the field of aqueous geochemistry.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404994_sm_miscellaneous_information.pdf3.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. L. Shock, E. H. Oelkers, J. W. Johnson, D. A. Sverjensky, H. C. Helgeson, J. Chem. Soc. Faraday Trans. 1992, 88, 803.
- 2D. A. Sverjensky, B. Harrison, D. Azzolini, Geochim. Cosmochim. Acta 2014, 129, 125.
- 3D. Pan, L. Spanu, B. Harrison, D. A. Sverjensky, G. Galli, Proc. Natl. Acad. Sci. USA 2013, 110, 6646.
- 4M. Mookherjee, H. Keppler, C. E. Manning, Geochim. Cosmochim. Acta 2014, 133, 128.
- 5N. Zotov, H. Keppler, Chem. Geol. 2002, 184, 71.
- 6B. O. Mysen, Am. Mineral. 2010, 95, 1807.
- 7B. O. Mysen, K. Mibe, I. M. Chou, W. A. Bassett, J. Geophys. Res. [Solid Earth] 2013, 118, 6076.
- 8J. D. Frantz, J. Dubessy, B. O. Mysen, Chem. Geol. 1994, 116, 181.
- 9S. Facq, I. Daniel, D. A. Sverjensky, Geochim. Cosmochim. Acta 2014, 132, 375.
- 10G. S. Pokrovski, L. S. Dubrovinsky, Science 2011, 331, 1052.
- 11C. Schmidt, R. Thomas, W. Heinrich, Geochim. Cosmochim. Acta 2005, 69, 275.
- 12A. J. Simpson, M. J. Simpson, R. Soong, Environ. Sci. Technol. 2012, 46, 11488.
- 13J. Jonas, P. Koziol, X. Peng, C. Reiner, D. M. Campbell, J. Magn. Reson. Ser. B 1993, 102, 299.
- 14L. Ballard, A. M. Yu, C. Reiner, J. Jonas, J. Magn. Reson. 1998, 133, 190.
- 15M. M. Hoffmann, M. S. Conradi, Rev. Sci. Instrum. 1997, 68, 159.
- 16R. L. Johnson, S. J. Harley, C. A. Ohlin, A. F. Panasci, W. H. Casey, ChemPhysChem 2011, 12, 2903.
- 17S. J. Harley, C. A. Ohlin, R. L. Johnson, A. F. Panasci, W. H. Casey, Angew. Chem. 2011, 123, 4559;
10.1002/ange.201006961 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4467.
- 18R. L. Johnson, C. A. Ohlin, K. Pellegrini, P. C. Burns, W. H. Casey, Angew. Chem. 2013, 125, 7612;
10.1002/ange.201301973 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 7464.
- 19M. Lesemann, K. Thirumoorthy, Y. J. Kim, J. Jonas, M. E. Paulaitis, Langmuir 1998, 14, 5339.
- 20J. Y. Buser, A. D. McFarland, Chem. Commun. 2014, 50, 4234.
- 21J. Jonas, L. Ballard, D. Nash, Biophys. J. 1998, 75, 445.
- 22C. E. Munte, M. B. Erlach, W. Kremer, J. Koehler, H. R. Kalbitzer, Angew. Chem. 2013, 125, 9111;
10.1002/ange.201301537 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 8943.
- 23J. Roche, J. Ying, A. S. Maltsev, A. Bax, ChemBioChem 2013, 14, 1754.
- 24H. R. Kalbitzer, I. C. Rosnizeck, C. E. Munte, S. P. Narayanan, V. Kropf, M. Spoerner, Angew. Chem. 2013, 125, 14492;
10.1002/ange.201305741 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 14242.
- 25I. R. Walker, Rev. Sci. Instrum. 1999, 70, 3402.
- 26K. Koyama-Nakazawa, M. Koeda, M. Hedo, Y. Uwatoko, Rev. Sci. Instrum. 2007, 78, 066109.
- 27G. J. Piermarini, S. Block, J. D. Barnett, R. A. Forman, J. Appl. Phys. 1975, 46, 2774.
- 28Z. A. Nalkina, Izu. Sib. Otd. Akad. Nauk SSSR Ser. Khim. Nauk 1983, 2, 46.
- 29M. Bishop, N. Shahid, J. Z. Yang, A. R. Barron, Dalton Trans. 2004, 2621.
- 30R. K. Momii, N. H. Nachtrieb, Inorg. Chem. 1967, 6, 1189.
- 31C. G. Salentine, Inorg. Chem. 1983, 22, 3920.
- 32K. E. Bett, J. B. Cappi, Nature 1965, 207, 620.
- 33L. Babcock, R. Pizer, Inorg. Chem. 1980, 19, 56.
- 34R. Pizer, L. Babcock, Inorg. Chem. 1977, 16, 1677.
- 35L. Babcock, R. Pizer, Inorg. Chem. 1983, 22, 174.
- 36R. Pizer, P. J. Ricatto, S. Jacobson, Inorg. Chem. 1995, 34, 1007.
- 37M. Pasdeloup, C. Brisson, Org. Magn. Reson. 1981, 16, 164.
- 38R. Pizer, P. J. Ricatto, Inorg. Chem. 1994, 33, 2402.
- 39T. Okamoto, A. Tanaka, E. Watanabe, T. Miyazaki, T. Sugaya, S. Iwatsuki, M. Inamo, H. D. Takagi, A. Odani, K. Ishihara, Eur. J. Inorg. Chem. 2014, 2389–2395.
- 40F. Bloch, W. W. Hansen, M. Packard, Phys. Rev. 1946, 70, 474.
- 41H. M. McConnell, J. Chem. Phys. 1958, 28, 430.
- 42C. D. Hubbard, R. van Eldik in Physical Inorganic Chemistry: Principles, Methods, and Models (Ed.: ), Wiley, Hoboken, 2010, p. 269.
10.1002/9780470602539.ch7 Google Scholar
- 43A. Drljaca, C. D. Hubbard, R. van Eldik, T. Asano, M. V. Basilevsky, W. J. le Noble, Chem. Rev. 1998, 98, 2167.
- 44M. Choukroun, O. Grasset, J. Chem. Phys. 2007, 127, 124506.