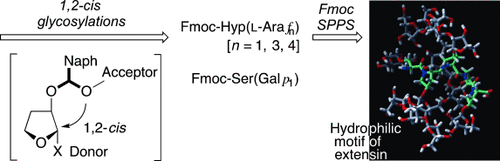Synthesis of the Highly Glycosylated Hydrophilic Motif of Extensins†
Corresponding Author
Dr. Akihiro Ishiwata
Synthetic Cellular Chemistry Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)
Synthetic Cellular Chemistry Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)Search for more papers by this authorCorresponding Author
Dr. Yukishige Ito
Synthetic Cellular Chemistry Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)
ERATO glycotrilogy project, JST (Japan)
Synthetic Cellular Chemistry Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)Search for more papers by this authorCorresponding Author
Dr. Akihiro Ishiwata
Synthetic Cellular Chemistry Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)
Synthetic Cellular Chemistry Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)Search for more papers by this authorCorresponding Author
Dr. Yukishige Ito
Synthetic Cellular Chemistry Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)
ERATO glycotrilogy project, JST (Japan)
Synthetic Cellular Chemistry Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)Search for more papers by this authorWe thank Dr. Hiroyuki Koshino (RIKEN Global Research Cluster) and his staff for technical help for technical help with ESI MS and CD, and Dr. Fumiaki Hayashi and Dr. Hui-ping Zhang (RIKEN Center for Life Science Technologies) for 900 MHz NMR measurements and are grateful to the Support Unit for Biomaterial Analysis (RIKEN Brain Science Institute) for tandem MS analysis. We also thank Ms. Akemi Takahashi for her kind technical assistance. This work was partly supported by Incentive Research Grant (2013) in RIKEN.
Graphical Abstract
Just a phase: Stereoselective synthesis of one of the highly glycosylated hydrophilic motifs of extensins has been completed. Key steps were a 2-naphthylmethyl ether-mediated intramolecular aglycon delivery to the stereoselective construction of the Ser(Galp1) and Hyp(Arafn) (n=1, 3, 4) fragments and Fmoc solid-phase peptide synthesis (SPPS) for the highly glycosylated pentapeptide motif.
Abstract
Extensin, the structural motif of plant extracellular matrix proteins, possesses a unique highly glycosylated, hydrophilic, and repeating Ser1Hyp4 pentapeptide unit, and has been proposed to include post-translational hydroxylation at proline residue and subsequent oligo-L-arabinosylations at all of the resultant hydroxyprolines as well as galactosylation at serine residue. Reported herein is the stereoselective synthesis of one of the highly glycosylated motifs, Ser(Galp1)-Hyp(Araf4)-Hyp(Araf4)-Hyp(Araf3)-Hyp(Araf1). The synthesis has been completed by the application of 2-(naphthyl)methylether-mediated intramolecular aglycon delivery to the stereoselective construction of the Ser(Galp1) and Hyp(Arafn) fragments as the key step, as well as Fmoc solid-phase peptide synthesis for the backbone pentapeptide.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404904_sm_miscellaneous_information.pdf3.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews, see: M. J. Kieliszewski, D. T. A. Lamport, L. Tan, M. C. Cannon in Ann. Plant Rev.: Plant Polysaccharides, Biosynthesis and Bioengineering, Vol. 41 (Ed.: ), Wiley-Blackwell, Oxford, 2011, pp. 321–342, and references therein.
- 2
- 2aR. Hieta, J. Myllyharju, J. Biol. Chem. 2002, 277, 23965–23971;
- 2bP. Tiainen, J. Myllyharju, P. Koivunen, J. Biol. Chem. 2005, 280, 1142–1148.
- 3B. L. Petersen, K. Faber, P. Ulvskov in Ann. Plant Rev.: Plant Polysaccharides, Biosynthesis and Bioengineering, Vol. 41 (Ed.: ), Wiley-Blackwell, Oxford, 2011, pp. 305–320.
- 4S. M. Velasquez, et al, Science 2011, 332, 1401–1403.
- 5
- 5aY. Matsubayashi, Plant Cell Physiol. 2011, 52, 5–13;
- 5bY. Matsubayashi, Genes Cells 2012, 17, 1–10.
- 6K. Ohyama, H. Shinohara, M. Ogawa-Ohnishi, Y. Matsubayashi, Nat. Chem. Biol. 2009, 5, 578–580.
- 7D. T. A. Lamport, L. Katona, S. Roerig, Biochem. J. 1973, 133, 125–131.
- 8M. J. Kieliszewski, D. T. A. Lamport, Plant J. 1994, 5, 157–172.
- 9M. C. Cannon, K. Terneus, Q. Hall, L. Tan, Y. Wang, B. L. Wegenhart, L. Chen, D. T. A. Lamport, Y. Chen, M. J. Kieliszewski, Proc. Natl. Acad. Sci. USA 2008, 105, 2226–2231.
- 10S. C. Fry, Biochem. J. 1982, 204, 449–455.
- 11L. Epstein, D. T. A. Lamport, Phytochemistry 1984, 23, 1241–1246.
- 12J. M. Estévez, M. J. Kieliszewski, N. Khitrov, C. Somerville, Plant Physiol. 2006, 142, 458–470.
- 13S. Gille, U. Hänsel, M. Ziemann, M. Pauly, Proc. Natl. Acad. Sci. USA 2009, 106, 14699–14704.
- 14
- 14aY. Akiyama, K. Kato, Agric. Biol. Chem. 1976, 40, 2343–2348;
- 14bY. Akiyama, K. Kato, Agric. Biol. Chem. 1977, 41, 79–81.
- 15
- 15aF. M. Klis, H. Eeltink, Planta 1979, 144, 479–484;
- 15bD. Ashford, N. N. Desai, A. K. Allen, A. Neuberger, M. A. O’Neil, R. R. Selvendran, Biochem. J. 1982, 201, 199–208.
- 16
- 16aK. Fujita, Y. Takashi, E. Obuchi, K. Kitahara, T. Suganuma, J. Biol. Chem. 2014, 289, 6110–6119;
- 16bK. Fujita, S. Sakamoto, Y. Ono, M. Wakao, Y. Suda, K. Kitahara, T. Suganuma, J. Biol. Chem. 2011, 286, 5143–5150.
- 17M. Ogawa-Ohnishi, W. Matsushita, Y. Matsubayashi, Nat. Chem. Biol. 2013, 9, 726–730.
- 18T. Ito, K. Saikawa, S. Kim, K. Fujita, A. Ishiwata, S. Kaeothip, T. Arakawa, T. Wakagi, G. T. Beckham, Y. Ito, S. Fushinobu, Biochen. Biophys. Res. Commun. 2014, 447, 32–37.
- 19For recent reviews on 1,2-cis glycosylation, see:
- 19aA. Ishiwata, Y. Ito, Trends Glycosci. Glycotechnol. 2009, 21, 266–289;
- 19bL. K. Mydock, A. V. Demchenko, Org. Biomol. Chem. 2010, 8, 497–510.
- 20For recent reviews, see:
- 20aA. Imamura, T. L. Lowary, Trends Glycosci. Glycotechnol. 2011, 23, 134–152; For our studies on intermolecular approach, see:
- 20bA. Ishiwata, H. Akao, Y. Ito, Org. Lett. 2006, 8, 5525–5528;
- 20cA. Ishiwata, H. Akao, Y. Ito, M. Sunagawa, N. Kusunose, Y. Kashiwazaki, Bioorg. Med. Chem. 2006, 14, 3049–3061.
- 21For PMB-ether-mediated IAD, see:
- 21aY. Ito, T. Ogawa, Angew. Chem. 1994, 106, 1843–1845; Angew. Chem. Int. Ed. Engl. 1994, 33, 1765–1767;
- 21bJ. Désiré, J. Prandi, Carbohydr. Res. 1999, 317, 110–118;
- 21cH. Shinohara, Y. Matsubayashi, Plant Cell Physiol. 2013, 54, 369–374;
- 21dS. Okamoto, H. Shinohara, T. Mori, Y. Matsubayashi, M. Kawaguchi, Nat. Commun. 2013, 4, 2191.
- 22
- 22aA. Ishiwata, Y. Munemura, Y. Ito, Eur. J. Org. Chem. 2008, 4250–4263;
- 22bA. Ishiwata, Y. Ito, J. Am. Chem. Soc. 2011, 133, 2275–2291.
- 23N. Xie, C. M. Taylor, Org. Lett. 2010, 12, 4968–4971.
- 24S. Kaeothip, G.-J. Boons, Org. Biomol. Chem. 2013, 11, 5136–5146.
- 25For recent examples, see:
- 25aT. Tsujimoto, Y. Ito, Tetrahedron Lett. 2007, 48, 5513–5516;
- 25bL.-D. Huang, H.-J. Lin, P.-H. Huang, W.-C. Hsiao, V. R. Reddy, S.-L. Fu, C.-C. Lin, Org. Biomol. Chem. 2011, 9, 2492–2504;
- 25cY. Kim, K. Oh, S. Song, D.-S. Lee, S. B. Park, J. Med. Chem. 2013, 56, 7100–7109.
- 26For recent reviews, see:
- 26aI. Cumpstey, Carbohydr. Res. 2008, 343, 1553–1573;
- 26bA. T. Carmona, A. J. Moreno-Vargas, I. Robina, Curr. Org. Synth. 2008, 5, 33–63;
- 26cA. Ishiwata, Y. J. Lee, Y. Ito, Org. Biomol. Chem. 2010, 8, 3596–3608.
- 27A. Ishiwata, Y. Ito, Yuki Gosei Kagaku Kyokaishi 2012, 70, 382–394.
- 28For 1,2-cis pyranosylation, see:
- 28aY. J. Lee, A. Ishiwata, Y. Ito, J. Am. Chem. Soc. 2008, 130, 6330–6331;
- 28bA. Ishiwata, A. Sakurai, Y. Nishimiya, S. Tsuda, Y. Ito, J. Am. Chem. Soc. 2011, 133, 19524–19535.
- 29S. Kaeothip, A. Ishiwata, Y. Ito, Org. Biomol. Chem. 2013, 11, 5892–5907.
- 30S. Kaeothip, A. Ishiwata, T. Ito, S. Fushinobu, K. Fujita, Y. Ito, Carbohydr. Res. 2013, 382, 95–100.
- 31See the Supporting Information.
- 32A. El-Faham, R. S. Funosas, R. Prohens F. Albericio, Chem. Eur. J. 2009, 15, 9404–9416.
- 33W. Lukenmaier, H. Zahn, Justus Liebigs Ann. Chem. 1970, 740, 1–17.
- 34P. Rovero, S. Viganò, S. Pegoraro, L. Quartara, Lett. Pept. Sci. 1995, 2, 319–323.
10.1007/BF00119994 Google Scholar
- 35J. L. Lauer, C. G. Fields, G. B. Fields, Lett. Pept. Sci. 1994, 1, 197–205.
- 36M. A. Fara, J. J. Diaz-Mochon, M. Bradley, Tetrahedron Lett. 2006, 47, 1011–1014.
- 37U. Westerlind, A. Hobel, N. Gaidzik, E. Schmitt, H. Kunz, Angew. Chem. 2008, 120, 7662–7667;
10.1002/ange.200802102 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7551–7556.
- 38C. M. Deber, F. A. Bovey, J. P. Carver, E. R. Blout, J. Am. Chem. Soc. 1970, 92, 6191–6198.
- 39
- 39aN. W. Owens, C. Braun, J. D. O’Neil, K. Marat, F. Schweizer, J. Am. Chem. Soc. 2007, 129, 11670–11671;
- 39bN. W. Owens, A. Lee, K. Marat, F. Schweizer, Chem. Eur. J. 2009, 15, 10649–10657.