Synthesis of Bridged Oxafenestranes from Pleuromutilin†
Robert W. Hicklin
Department of Chemistry, University of Illinois at Urbana-Champaign, 261 RAL, Box 36-5, 600 S. Mathews, Urbana, IL 61801 (USA)
Search for more papers by this authorTania L. López Silva
Department of Chemistry, University of Illinois at Urbana-Champaign, 261 RAL, Box 36-5, 600 S. Mathews, Urbana, IL 61801 (USA)
Search for more papers by this authorCorresponding Author
Prof. Paul J. Hergenrother
Department of Chemistry, University of Illinois at Urbana-Champaign, 261 RAL, Box 36-5, 600 S. Mathews, Urbana, IL 61801 (USA)
Department of Chemistry, University of Illinois at Urbana-Champaign, 261 RAL, Box 36-5, 600 S. Mathews, Urbana, IL 61801 (USA)Search for more papers by this authorRobert W. Hicklin
Department of Chemistry, University of Illinois at Urbana-Champaign, 261 RAL, Box 36-5, 600 S. Mathews, Urbana, IL 61801 (USA)
Search for more papers by this authorTania L. López Silva
Department of Chemistry, University of Illinois at Urbana-Champaign, 261 RAL, Box 36-5, 600 S. Mathews, Urbana, IL 61801 (USA)
Search for more papers by this authorCorresponding Author
Prof. Paul J. Hergenrother
Department of Chemistry, University of Illinois at Urbana-Champaign, 261 RAL, Box 36-5, 600 S. Mathews, Urbana, IL 61801 (USA)
Department of Chemistry, University of Illinois at Urbana-Champaign, 261 RAL, Box 36-5, 600 S. Mathews, Urbana, IL 61801 (USA)Search for more papers by this authorWe are grateful to the University of Illinois at Urbana-Champaign for support of this work. We would like to thank Dr. Danielle Gray and Dr. Jeffrey Bertke for X-ray analysis.
Graphical Abstract
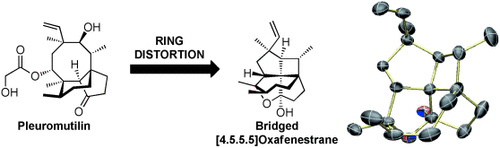
Fenestranes are highly strained molecules possessing a quaternary carbon atom with bonds that deviate from the canonical tetrahedral geometry. The natural product pleuromutilin can be used as a starting material for the synthesis of bridged [4.5.5.5]- and [4.5.7.5]oxafenestranes through a carbocation rearrangement cascade. X-ray crystallography shows that these compounds exhibit significant planarization of the central tetracoordinate carbon center.
Abstract
Fenestranes are an intriguing class of highly strained molecules possessing a quaternary carbon with bonds that deviate from the canonical tetrahedral geometry. Herein we report the discovery that the natural product pleuromutilin can be used as a structurally complex starting material for the synthesis of a series of bridged cis,cis,cis,cis-[4.5.5.5]- and cis,cis,cis,cis-[4.5.7.5]oxafenestranes through a carbocation rearrangement cascade. X-ray crystallographic analysis of several cis,cis,cis,cis-[4.5.5.5]oxafenestranes shows a significant planarization of the central tetracoordinate carbon atom and demonstrates the influence of bridgehead substituents and bridging rings on planarity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404765_sm_miscellaneous_information.pdf2.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. H. van ‘t Hoff, Arch. Neerl. Sci. Exactes Nat. 1874, 9, 44;
- 1bJ. A. Le Bel, Bul. Soc. Chim. Fr. 1874, 22, 337.
- 2V. I. Minkin, R. M. Minyaev, R. Hoffmann, Russ. Chem. Rev. 2002, 71, 869–892.
- 3For reviews see:
- 3aA. Boudhar, M. Charpenay, G. Blond, J. Suffert, Angew. Chem. 2013, 125, 13020–13032;
10.1002/ange.201304555 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 12786–12798;
- 3bR. Keese, Chem. Rev. 2006, 106, 4787–4808;
- 3cD. Kuck, Chem. Rev. 2006, 106, 4885–4925;
- 3dB. R. Venepalli, W. C. Agosta, Chem. Rev. 1987, 87, 399–410.
- 4R. E. Corbett, D. R. Lauren, R. T. Weavers, J. Chem. Soc. Perkin Trans. 1 1979, 1774–1790.
- 5S. H. Shim, J. B. Gloer, D. T. Wicklow, J. Nat. Prod. 2006, 69, 1601–1605.
- 6N. Ingavat, C. Mahidol, S. Ruchirawat, P. Kittakoop, J. Nat. Prod. 2011, 74, 1650–1652.
- 7
- 7aV. Georgian, M. Saltzman, Tetrahedron Lett. 1972, 13, 4315–4317;
10.1016/S0040-4039(01)94304-7 Google Scholar
- 7bM. N. Deshpande, M. Jawdosiuk, G. Kubiak, M. Venkatachalam, U. Weiss, J. M. Cook, J. Am. Chem. Soc. 1985, 107, 4786–4788;
- 7cV. B. Rao, C. F. George, S. Wolff, W. C. Agosta, J. Am. Chem. Soc. 1985, 107, 5732–5739;
- 7dP. A. Wender, T. W. Von Geldern, B. H. Levine, J. Am. Chem. Soc. 1988, 110, 4858–4860;
- 7eM. Thommen, P. Gerber, R. Keese, Chimia 1991, 45, 21–24;
- 7fX. Fu, G. Kubiak, W. Zhang, W. Han, A. K. Gupta, J. M. Cook, Tetrahedron 1993, 49, 1511–1524;
- 7gJ. Wang, R. Guidetti-Grept, R. Keese, H. Stoeckli-Evans, Helv. Chim. Acta 1997, 80, 1169–1175;
- 7hC. Hulot, G. Blond, J. Suffert, J. Am. Chem. Soc. 2008, 130, 5046–5047;
- 7iM. Thommen, L. Prevot, M. K. Eberle, P. Bigler, R. Keese, Tetrahedron 2011, 67, 3868–3873.
- 8
- 8aT. Gaich, J. Mulzer, J. Am. Chem. Soc. 2008, 130, 452–453;
- 8bT. Gaich, J. Mulzer, Org. Lett. 2009, 11, 272–275;
- 8cC. S. Penkett, J. A. Woolford, I. J. Day, M. P. Coles, J. Am. Chem. Soc. 2010, 132, 4–5;
- 8dN. Heinrich, A. C. Willis, I. A. Cade, J. Ho, M. L. Coote, M. G. Banwell, Chem. Eur. J. 2012, 18, 13585–13588;
- 8eW. Chen, J.-H. Tay, J. Ying, X.-Q. Yu, L. Pu, J. Org. Chem. 2013, 78, 2256–2265.
- 9
- 9aS. E. Denmark, L. A. Kramps, J. I. Montgomery, Angew. Chem. 2002, 114, 4296–4299;
10.1002/1521-3757(20021104)114:21<4296::AID-ANGE4296>3.0.CO;2-4 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4122–4125;10.1002/1521-3773(20021104)41:21<4122::AID-ANIE4122>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 9bS. E. Denmark, J. I. Montgomery, Angew. Chem. 2005, 117, 3798–3802;
10.1002/ange.200500367 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3732–3736;
- 9cS. E. Denmark, J. I. Montgomery, L. A. Kramps, J. Am. Chem. Soc. 2006, 128, 11620–11630.
- 10D. Frense, Appl. Microbiol. Biotechnol. 2007, 73, 1233–1240.
- 11F.-M. Arcamone in Analogue-Based Drug Discovery II, Wiley-VCH, Weinheim, 2010, pp. 217–241.
10.1002/9783527630035.ch9 Google Scholar
- 12C. J. Paddon, P. J. Westfall, D. J. Pitera, K. Benjamin, K. Fisher, D. McPhee, M. D. Leavell, A. Tai, A. Main, D. Eng, D. R. Polichuk, K. H. Teoh, D. W. Reed, T. Treynor, J. Lenihan, M. Fleck, S. Bajad, G. Dang, D. Dengrove, D. Diola, G. Dorin, K. W. Ellens, S. Fickes, J. Galazzo, S. P. Gaucher, T. Geistlinger, R. Henry, M. Hepp, T. Horning, T. Iqbal, H. Jiang, L. Kizer, B. Lieu, D. Melis, N. Moss, R. Regentin, S. Secrest, H. Tsuruta, R. Vazquez, L. F. Westblade, L. Xu, M. Yu, Y. Zhang, L. Zhao, J. Lievense, P. S. Covello, J. D. Keasling, K. K. Reiling, N. S. Renninger, J. D. Newman, Nature 2013, 496, 528–532.
- 13D. J. Newman, G. M. Cragg, J. Nat. Prod. 2012, 75, 311–335.
- 14H. Renata, Q. Zhou, P. S. Baran, Science 2013, 339, 59–63.
- 15A. Giannis, P. Heretsch, V. Sarli, A. Stößel, Angew. Chem. 2009, 121, 8052–8055;
10.1002/ange.200902520 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7911–7914.
- 16K.-G. Fahlbusch, F.-J. Hammerschmidt, J. Panten, W. Pickenhagen, D. Schatkowski, K. Bauer, D. Garbe, H. Surburg, Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000, pp. 143–144.
- 17K. C. Morrison, P. J. Hergenrother, Nat. Prod. Rep. 2014, 31, 6–14.
- 18
- 18aR. W. Huigens III, K. C. Morrison, R. W. Hicklin, T. A. Flood, Jr., M. F. Richter, P. J. Hergenrother, Nat. Chem. 2013, 5, 195–202;
- 18bR. J. Rafferty, R. W. Hicklin, K. A. Maloof, P. J. Hergenrother, Angew. Chem. 2014, 126, 224–228;
10.1002/ange.201308743 Google ScholarAngew. Chem. Int. Ed. 2014, 53, 220–224.
- 19
- 19aF. Kavanagh, A. Hervey, W. J. Robbins, Proc. Natl. Acad. Sci. USA 1951, 48, 570–574;
- 19bY.-Z. Tang, Y.-H. Liu, J.-X. Chen, Mini-Rev. Med. Chem. 2012, 12, 53–61.
- 20R. Novak, Ann. N. Y. Acad. Sci. 2011, 1241, 71–81.
- 21A. J. Birch, C. W. Holzapfel, R. W. Rickards, Tetrahedron 1966, 22, 359–387.
10.1016/S0040-4020(01)90949-4 Google Scholar
- 22
- 22aH. Wang, Y. W. Andemichael, F. G. Vogt, J. Org. Chem. 2008, 73, 478–481;
- 22bG. Brooks, W. Burgess, D. Colthurst, J. D. Hinks, E. Hunt, M. J. Pearson, B. Shea, A. K. Takle, J. M. Wilson, G. Woodnutt, Bioorg. Med. Chem. 2001, 9, 1221–1231.
- 23M. Thommen, R. Keese, M. Fortsch, Acta Crystallogr. Sect. C 1996, 52, 2051–2053.
- 24M. Charpenay, A. Boudhar, G. Blond, J. Suffert, Angew. Chem. 2012, 124, 4455–4458;
10.1002/ange.201107934 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4379–4382.
- 25
- 25aZ. Wang in Comprehensive Organic Name Reactions and Reagents, Wiley, Hoboken, 2010;
10.1002/9780470638859 Google Scholar
- 25bA. G. Myers, M. Siu, F. Ren, J. Am. Chem. Soc. 2002, 124, 4230–4232.