An Unusual Protein–Protein Interaction through Coupled Unfolding and Binding†
This work was supported by a National Research Foundation of Korea (NRF) grant (2013R1A1A2010856). We thank the high-field NMR facility at the Korea Basic Science Institute and the National Center for Inter-University Research Facilities.
Graphical Abstract
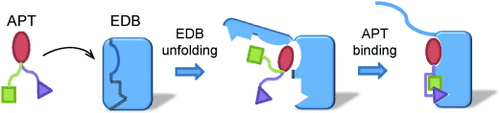
Unfold and hold: It is known that protein–protein interactions can involve coupled folding and binding, but coupled unfolding and binding is not well characterized. An unusual protein–protein interaction is described in which the binding of an aptide (APT) to fibronectin extradomain B (EDB) involves partial unfolding to expose the binding surface. The structural and energetic details were determined by NMR spectroscopy and thermodynamic analysis.
Abstract
Aptides, a novel class of high-affinity peptides, recognize diverse molecular targets with high affinity and specificity. The solution structure of the aptide APT specifically bound to fibronectin extradomain B (EDB), which represents an unusual protein–protein interaction that involves coupled unfolding and binding, is reported. APT binding is accompanied by unfolding of the C-terminal β strand of EDB, thereby permitting APT to interact with the freshly exposed hydrophobic interior surfaces of EDB. The β-hairpin scaffold of APT drives the interaction by a β-strand displacement mechanism, such that an intramolecular β sheet is replaced by an intermolecular β sheet. The unfolding of EDB perturbs the tight domain association between EDB and FN8 of fibronectin, thus highlighting its potential use as a scaffold that switches between stretched and bent conformations.