Plasmon-Induced Ammonia Synthesis through Nitrogen Photofixation with Visible Light Irradiation†
This study was supported by funding from the Ministry of Education, Culture, Sports, Science, and Technology of Japan: KAKENHI Grant-in-Aid for Scientific Research (s) (no. 23225006), Grant-in-Aid for Research Activity Start-up (no. 25888002) and the Innovative Areas “Artificial Photosynthesis (AnApple)” (no. 25107501) grant from the Japan Society for the Promotion of Science (JSPS), the Nanotechnology Platform (Hokkaido University), and the Low-Carbon Research Network of Japan.
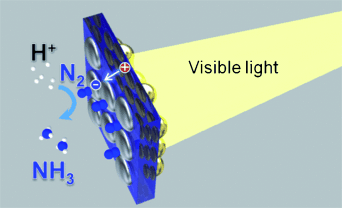
Graphical Abstract
See the light of day: A plasmon-induced ammonia synthesis technique that responds to visible light and is based on a strontium titanate (SrTiO3) photoelectrode loaded with gold nanoparticles has been developed. It is deduced that plasmon-induced charge separation at the Au/SrTiO3 interface promotes oxidation in the anodic chamber and subsequent nitrogen reduction on the cathodic side.
Abstract
We have successfully developed a plasmon-induced technique for ammonia synthesis that responds to visible light through a strontium titanate (SrTiO3) photoelectrode loaded with gold (Au) nanoparticles. The photoelectrochemical reaction cell was divided into two chambers to separate the oxidized (anodic side) and reduced (cathodic side) products. To promote NH3 formation, a chemical bias was applied by regulating the pH value of these compartments, and ethanol was added to the anodic chamber as a sacrificial donor. The quantity of NH3 formed at the ruthenium surface, which was used as a co-catalyst for SrTiO3, increases linearly as a function of time under irradiation with visible light at wavelengths longer than 550 nm. The NH3 formation action spectrum approximately corresponds to the plasmon resonance spectrum. We deduced that plasmon-induced charge separation at the Au/SrTiO3 interface promotes oxidation at the anodic chamber and subsequent nitrogen reduction on the cathodic side.