Unprecedented Role of Hydronaphthoquinone Tautomers in Biosynthesis†
Financial support of this work by the DFG (IRTG 1038) is gratefully acknowledged. We thank O. Fuchs for technical support, V. Brecht and S. Ferlaino for measurement of NMR spectra, Prof. A. Stolz, University of Stuttgart, for providing NfsB, 15 and Prof. G. Fuchs, University of Freiburg, for critically reading this paper. We acknowledge the use of the computing resources provided by the Black Forest Grid Initiative.
Graphical Abstract
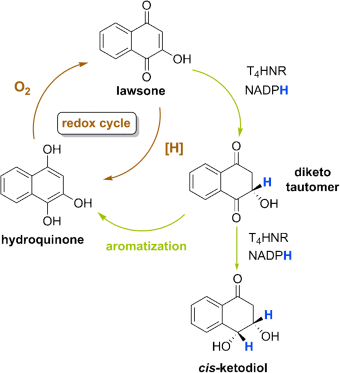
Breaking the cycle: In studies on the reduction of 2-hydroxynaphthoquinones to the stable 1,4-diketo tautomeric form of hydronaphthoquinones and their further reduction by fungal tetrahydroxynaphthalene reductase, diketo tautomers emerge as true intermediates in biosynthesis. Their formation breaks the (redox) cycle, thus protecting the cell from stress-related redox events.
Abstract
Quinones and hydroquinones are among the most common cellular cofactors, redox mediators, and natural products. Here, we report on the reduction of 2-hydroxynaphthoquinones to the stable 1,4-diketo tautomeric form of hydronaphthoquinones and their further reduction by fungal tetrahydroxynaphthalene reductase. The very high diastereomeric and enantiomeric excess, together with the high yield of cis-3,4-dihydroxy-1-tetralone, exclude an intermediary hydronaphthoquinone. Labeling experiments with NADPH and NADPD corroborated the formation of an unexpected 1,4-diketo tautomeric form of 2-hydroxyhydronaphthoquinone as a stable intermediate. Similar 1,4-diketo tautomers of hydronaphthoquinones were established as products of the NADPH-dependent enzymatic reduction of other 1,4-naphthoquinones, and as substrates for different members of the superfamily of short-chain dehydrogenases. We propose an essential role of hydroquinone diketo tautomers in biosynthesis and detoxification processes.