Fluorescence Sensing of Amine Vapors Using a Cationic Conjugated Polymer Combined with Various Anions†
Dr. Sébastien Rochat
Department of Chemistry and The Institute for Soldier Nanotechnologies, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Timothy M. Swager
Department of Chemistry and The Institute for Soldier Nanotechnologies, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Department of Chemistry and The Institute for Soldier Nanotechnologies, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)Search for more papers by this authorDr. Sébastien Rochat
Department of Chemistry and The Institute for Soldier Nanotechnologies, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Timothy M. Swager
Department of Chemistry and The Institute for Soldier Nanotechnologies, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Department of Chemistry and The Institute for Soldier Nanotechnologies, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)Search for more papers by this authorThis research was supported by the Army Research Office through MIT’s Institute for Soldier Nanotechnologies. S.R. acknowledges financial support from the Swiss National Science Foundation (Advanced Postdoctoral fellowship PA00P2-145389). We thank J. F. Fennell and Dr. J. Im for help with the XPS analyses.
Graphical Abstract
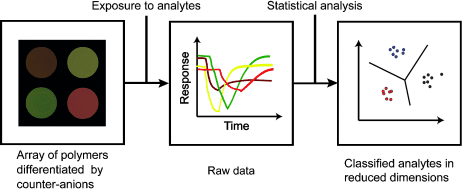
A sensor array comprising a conjugated cationic polymer combined with various counteranions has been developed. This simple approach allows the creation of polymer formulations able to detect vapors of industrially relevant amines in low ppm concentrations by fluorescence quenching measurements. Furthermore the array's response is useful to identify the nature of the analyte through pattern-based recognition algorithms.
Abstract
A series of conjugated cationic polymers, differentiated only by their accompanying counter-anions, was prepared and characterized. The choice of counter-anion (CA) was found to drastically impact the solubility of the polymers and their optical properties in solution and in the solid state. Fluorescent polymer thin films were found to be instantaneously quenched by volatile amines in the gas phase at low ppm concentrations, and a mini-array with CAs as variable elements was found to be able to differentiate amines with good fidelity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404439_sm_miscellaneous_information.pdf1.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz, A. B. Holmes, Chem. Rev. 2009, 109, 897–1091;
- 1bC. Wang, H. Dong, W. Hu, Y. Liu, D. Zhu, Chem. Rev. 2012, 112, 2208–2267;
- 1cX. Zhao, X. Zhan, Chem. Soc. Rev. 2011, 40, 3728–3743;
- 1dP. M. Beaujuge, J. M. J. Fréchet, J. Am. Chem. Soc. 2011, 133, 20009–20029;
- 1eL. Biniek, B. C. Schroeder, C. B. Nielsen, I. McCulloch, J. Mater. Chem. 2012, 22, 14803–14813.
- 2C. Li, M. Liu, N. G. Pschirer, M. Baumgarten, K. Müllen, Chem. Rev. 2010, 110, 6817–6855.
- 3
- 3aC. Zhu, L. Liu, Q. Yang, F. Lv, S. Wang, Chem. Rev. 2012, 112, 4687–4735;
- 3bP. Lin, F. Yan, Adv. Mater. 2012, 24, 34–51.
- 4
- 4aS. Rochat, T. M. Swager, ACS Appl. Mater. Interfaces 2013, 5, 4488–4502;
- 4bS. W. Thomas, G. D. Joly, T. M. Swager, Chem. Rev. 2007, 107, 1339–1386;
- 4cT. M. Swager, Acc. Chem. Res. 1998, 31, 201–207.
- 5
- 5aH.-A. Ho, A. Najari, M. Leclerc, Acc. Chem. Res. 2008, 41, 168–178;
- 5bM. Leclerc, Adv. Mater. 1999, 11, 1491–1498;
10.1002/(SICI)1521-4095(199912)11:18<1491::AID-ADMA1491>3.0.CO;2-O CAS Web of Science® Google Scholar
- 5cA. Duarte, K.-Y. Pu, B. Liu, G. C. Bazan, Chem. Mater. 2011, 23, 501–515;
- 5dJ. Liang, K. Li, B. Liu, Chem. Sci. 2013, 4, 1377–1394;
- 5eC. V. Hoven, A. Garcia, G. C. Bazan, T.-Q. Nguyen, Adv. Mater. 2008, 20, 3793–3810;
- 5fY. Liu, K. Ogawa, K. S. Schanze, J. Photochem. Photobiol. C 2009, 10, 173–190;
- 5gX. Duan, L. Liu, F. Feng, S. Wang, Acc. Chem. Res. 2010, 43, 260–270.
- 6
- 6aJ.-S. Yang, T. M. Swager, J. Am. Chem. Soc. 1998, 120, 11864–11873;
- 6bL. Feng, H. Li, Y. Qu, C. Lü, Chem. Commun. 2012, 48, 4633–4635;
- 6cL.-H. Zhang, T. Jiang, L.-B. Wu, J.-H. Wan, C.-H. Chen, Y.-B. Pei, H. Lu, Y. Deng, G.-F. Bian, H.-Y. Qiu, G.-Q. Lai, Chem. Asian J. 2012, 7, 1583–1593;
- 6dG. He, N. Yan, J. Yang, H. Wang, L. Ding, S. Yin, Y. Fang, Macromolecules 2011, 44, 4759–4766;
- 6eJ. C. Sanchez, W. C. Trogler, J. Mater. Chem. 2008, 18, 3143–3156;
- 6fL. Chen, D. W. McBranch, H.-L. Wang, R. Helgeson, F. Wudl, D. G. Whitten, Proc. Natl. Acad. Sci. USA 1999, 96, 12287–12292.
- 7
- 7aS. Rochat, T. M. Swager, J. Am. Chem. Soc. 2013, 135, 17703–17706;
- 7bY. Kim, J. E. Whitten, T. M. Swager, J. Am. Chem. Soc. 2005, 127, 12122–12130.
- 8S. W. Thomas III, J. P. Amara, R. E. Bjork, T. M. Swager, Chem. Commun. 2005, 4572–4574.
- 9
- 9aA. I. Costa, H. D. Pinto, L. F. V. Ferreira, J. V. Prata, Sens. Actuators B 2012, 161, 702–713;
- 9bL. Zhu, C. Yang, W. Zhang, J. Qin, Polymer 2008, 49, 217–224;
- 9cB. Esser, T. M. Swager, Angew. Chem. Int. Ed. 2010, 49, 8872–8875; Angew. Chem. 2010, 122, 9056–9059.
- 10
- 10aT.-H. Kim, T. M. Swager, Angew. Chem. Int. Ed. 2003, 42, 4803–4806; Angew. Chem. 2003, 115, 4951–4954;
- 10bJ. H. Wosnick, C. M. Mello, T. M. Swager, J. Am. Chem. Soc. 2005, 127, 3400–3405.
- 11
- 11aR. Yang, A. Garcia, D. Korystov, A. Mikhailovsky, G. C. Bazan, T.-Q. Nguyen, J. Am. Chem. Soc. 2006, 128, 16532–16539;
- 11bC.-S. Tsai, S.-H. Yang, B.-C. Liu, H.-C. Su, Org. Electron. 2013, 14, 488–499.
- 12A. Garcia, Y. Jin, J. Z. Brzezinski, T.-Q. Nguyen, J. Phys. Chem. C 2010, 114, 22309–22315.
- 13J. H. Ortony, R. Yang, J. Z. Brzezinski, L. Edman, T.-Q. Nguyen, G. C. Bazan, Adv. Mater. 2008, 20, 298–302.
- 14M. Kang, O. K. Nag, R. R. Nayak, S. Hwang, H. Suh, H. Y. Woo, Macromolecules 2009, 42, 2708–2714.
- 15D. Izuhara, T. M. Swager, J. Am. Chem. Soc. 2009, 131, 17724–17725.
- 16
- 16aV. Gutmann, Coord. Chem. Rev. 1976, 18, 225–255;
- 16bF. Arnaud-Neu, R. Delgado, S. Chaves, Pure Appl. Chem. 2003, 75, 71–102.
- 17J. A. Osaheni, S. A. Jenekhe, J. Am. Chem. Soc. 1995, 117, 7389–7398.
- 18
- 18aP. Xue, Q. Xu, P. Gong, C. Qian, A. Ren, Y. Zhang, R. Lu, Chem. Commun. 2013, 49, 5838–5840;
- 18bJ. J. Peterson, A. R. Davis, M. Werre, E. B. Coughlin, K. R. Carter, ACS Appl. Mater. Interfaces 2011, 3, 1796–1799;
- 18cY. Che, X. Yang, Z. Zhang, J. Zuo, J. S. Moore, L. Zang, Chem. Commun. 2010, 46, 4127–4129;
- 18dY. Che, X. Yang, S. Loser, L. Zang, Nano Lett. 2008, 8, 2219–2223.
- 19Y. Kim, T. M. Swager, Macromolecules 2006, 39, 5177–5179.
- 20C. J. Cumming, C. Aker, M. Fisher, M. Fox, M. J. La Grone, D. Reust, M. G. Rockley, T. M. Swager, E. Towers, V. Williams, IEEE Trans. Geosci. Remote Sens. 2001, 39, 1119–1128.
- 21http://www.osha.gov (retrieved 10/28/2013).
- 22N. A. Rakow, K. S. Suslick, Nature 2000, 406, 710–713.
- 23J. R. Askim, M. Mahmoudi, K. S. Suslick, Chem. Soc. Rev. 2013, 42, 8649–8682.
- 24P. C. Jurs, G. A. Bakken, H. E. McClelland, Chem. Rev. 2000, 100, 2649–2678.
- 25S. Rochat, J. Gao, X. Qian, F. Zaubitzer, K. Severin, Chem. Eur. J. 2010, 16, 104–113.