Selective Photoelectrochemical Reduction of Aqueous CO2 to CO by Solvated Electrons†
This work was supported by the Air Force Office of Scientific Research under AFOSR Award FA9550-12-1-0063. L.Z. thanks Fei Meng for assistance of X-ray diffraction measurements, Steve Strebel, LeRoy Dobson, and Christopher McSweeny at Wisconsin State Laboratory of Hygiene for the assistance of ion chromatography measurements.
Graphical Abstract
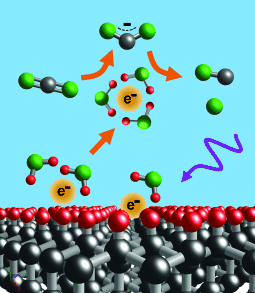
Diamond in the rough: Illumination of diamond substrates leads to emission of electrons into aqueous media. The solvated electrons are potent reducing agents and induce the direct one-electron reduction of CO2 to CO2.−, which then forms CO. This approach represents a new concept in catalysis by directly releasing electrons into reactant liquids.
Abstract
Reduction of CO2 by direct one-electron activation is extraordinarily difficult because of the −1.9 V reduction potential of CO2. Demonstrated herein is reduction of aqueous CO2 to CO with greater than 90 % product selectivity by direct one-electron reduction to CO2.− by solvated electrons. Illumination of inexpensive diamond substrates with UV light leads to the emission of electrons directly into water, where they form solvated electrons and induce reduction of CO2 to CO2.−. Studies using diamond were supported by studies using aqueous iodide ion (I−), a chemical source of solvated electrons. Both sources produced CO with high selectivity and minimal formation of H2. The ability to initiate reduction reactions by emitting electrons directly into solution without surface adsorption enables new pathways which are not accessible using conventional electrochemical or photochemical processes.