Asymmetric Hydroxylative Phenol Dearomatization Promoted by Chiral Binaphthylic and Biphenylic Iodanes†
Cyril Bosset
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
These authors contributed equally to this work.
Search for more papers by this authorRomain Coffinier
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
These authors contributed equally to this work.
Search for more papers by this authorDr. Philippe A. Peixoto
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
Search for more papers by this authorMourad El Assal
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
Search for more papers by this authorDr. Karinne Miqueu
Univ. Pau et Pays de l'Adour, IPREM (CNRS-UMR 5254), Hélioparc, 2 avenue Pierre Angot, 64053 Pau Cedex 09 (France)
Search for more papers by this authorDr. Jean-Marc Sotiropoulos
Univ. Pau et Pays de l'Adour, IPREM (CNRS-UMR 5254), Hélioparc, 2 avenue Pierre Angot, 64053 Pau Cedex 09 (France)
Search for more papers by this authorCorresponding Author
Dr. Laurent Pouységu
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)Search for more papers by this authorCorresponding Author
Prof. Stéphane Quideau
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)Search for more papers by this authorCyril Bosset
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
These authors contributed equally to this work.
Search for more papers by this authorRomain Coffinier
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
These authors contributed equally to this work.
Search for more papers by this authorDr. Philippe A. Peixoto
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
Search for more papers by this authorMourad El Assal
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
Search for more papers by this authorDr. Karinne Miqueu
Univ. Pau et Pays de l'Adour, IPREM (CNRS-UMR 5254), Hélioparc, 2 avenue Pierre Angot, 64053 Pau Cedex 09 (France)
Search for more papers by this authorDr. Jean-Marc Sotiropoulos
Univ. Pau et Pays de l'Adour, IPREM (CNRS-UMR 5254), Hélioparc, 2 avenue Pierre Angot, 64053 Pau Cedex 09 (France)
Search for more papers by this authorCorresponding Author
Dr. Laurent Pouységu
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)Search for more papers by this authorCorresponding Author
Prof. Stéphane Quideau
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)
Univ. Bordeaux, ISM (CNRS-UMR 5255) and IECB, 2 rue Robert Escarpit, 33607 Pessac Cedex (France)Search for more papers by this authorFinancial support from the Agence Nationale de la Recherche (ANR-10-BLAN-0721), the CNRS, the Conseil Régional d′Aquitaine, and the French Ministry of Research, including doctoral assistantships for C.B., R.C., and M.E.A., is gratefully acknowledged. The authors also wish to thank Dr. J. Brioche for his contribution to the elaboration of this research project.
Graphical Abstract
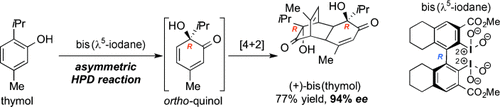
The selective oxygenation of iodobinaphthyls and iodobiphenyls afforded either λ3- or λ5-iodanes, which were evaluated for their capacity to promote asymmetric intermolecular hydroxylative phenol dearomatizations (HPDs). Most remarkably, a C2-symmetrical biphenylic λ5-iodane induced the HPD reaction/[4+2] cyclodimerization cascade of thymol into bis(thymol) with enantiomeric excesses of up to 94 %.
Abstract
The long-standing quest for chiral hypervalent organoiodine compounds (i.e., iodanes) as metal-free reagents for asymmetric synthesis continues. Although remarkable progress has recently been made in organoiodine-catalyzed reactions using a terminal oxidant in stoichiometric amounts, there is still a significant need for “flaskable” chiral iodane reagents. Herein, we describe the synthesis of new iodobinaphthyls and iodobiphenyls, their successful and selective DMDO-mediated oxidation into either λ3- or λ5-iodanes, and the evaluation of their capacity to promote asymmetric hydroxylative phenol dearomatization (HPD) reactions. Most notably, a C2-symmetrical biphenylic λ5-iodane promoted the HPD-induced conversion of the monoterpene thymol into the corresponding ortho-quinol-based [4+2] cyclodimer (i.e., bis(thymol)) with enantiomeric excesses of up to 94 %.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403571_sm_miscellaneous_information.pdf13.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For a selection of recent books and reviews on hypervalent iodine chemistry, see:
- 1aV. V. Zhdankin, Hypervalent Iodine Chemistry—Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds, Wiley, Chichester, 2013;
10.1002/9781118341155 Google Scholar
- 1bM. S. Yusubov, V. V. Zhdankin, Curr. Org. Synth. 2012, 9, 247;
- 1cA. Duschek, S. F. Kirsch, Angew. Chem. 2011, 123, 1562; Angew. Chem. Int. Ed. 2011, 50, 1524;
- 1dV. V. Zhdankin, J. Org. Chem. 2011, 76, 1185;
- 1eA. Varvoglis, Tetrahedron 2010, 66, 5739;
- 1fE. A. Merritt, B. Olofsson, Angew. Chem. 2009, 121, 9214; Angew. Chem. Int. Ed. 2009, 48, 9052;
- 1gV. V. Zhdankin, P. J. Stang, Chem. Rev. 2008, 108, 5299;
- 1hR. M. Moriarty, J. Org. Chem. 2005, 70, 2893;
- 1i Hypervalent Iodine Chemistry—Modern Developments in Organic Synthesis in Topics Curr. Chem., Vol. 224 (Ed.: ), Springer, Berlin, 2003.
- 2For recent reviews on the use of iodanes in synthesis, see:
- 2aR. Bernini, G. Fabrizi, L. Pouységu, D. Deffieux, S. Quideau, Curr. Org. Synth. 2012, 9, 650;
- 2bD. F. González, F. Benfatti, J. Waser, ChemCatChem 2012, 4, 955;
- 2cY. Kita, T. Dohi, K. Morimoto, J. Synth. Org. Chem. Jpn. 2011, 69, 1241;
- 2dL. F. Silva, Jr., B. Olofsson, Nat. Prod. Rep. 2011, 28, 1722;
- 2eE. A. Merritt, B. Olofsson, Synthesis 2011, 517;
- 2fL. Pouységu, D. Deffieux, S. Quideau, Tetrahedron 2010, 66, 2235;
- 2gM. Uyanik, K. Ishihara, Chem. Commun. 2009, 2086;
- 2hH. Tohma, Y. Kita, Adv. Synth. Catal. 2004, 346, 111.
- 3For reviews, highlights and a selection of recent publications on chiral iodanes and their use in organoiodine catalysis, see:
- 3aA. Parra, S. Reboredo, Chem. Eur. J. 2013, 19, 17244;
- 3bM. Uyanik, T. Yasui, K. Ishihara, Angew. Chem. 2013, 125, 9385;
10.1002/ange.201303559 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 9215;
- 3cT. Dohi, N. Takenaga, T. Nakae, Y. Toyoda, M. Yamasaki, M. Shiro, H. Fujioka, A. Maruyama, Y. Kita, J. Am. Chem. Soc. 2013, 135, 4558;
- 3dM. Shimogaki, M. Fujita, T. Sugimura, Eur. J. Org. Chem. 2013, 7128;
- 3eH. Liang, M. A. Ciufolini, Angew. Chem. 2011, 123, 12051; Angew. Chem. Int. Ed. 2011, 50, 11849;
- 3fT. Dohi, Y. Kita, Chem. Commun. 2009, 2073;
- 3gM. Ochiai, K. Miyamoto, Eur. J. Org. Chem. 2008, 4229;
- 3hR. D. Richardson, T. Wirth, Angew. Chem. 2006, 118, 4510; Angew. Chem. Int. Ed. 2006, 45, 4402 and references therein.
- 4
- 4aS. Quideau, G. Lyvinec, M. Marguerit, K. Bathany, A. Ozanne-Beaudenon, T. Buffeteau, D. Cavagnat, A. Chénedé, Angew. Chem. 2009, 121, 4675;
10.1002/ange.200901039 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4605;
- 4bL. Pouységu, T. Sylla, T. Garnier, L. B. Rojas, J. Charris, D. Deffieux, S. Quideau, Tetrahedron 2010, 66, 5908;
- 4cIn the absence of the iodoarene A, we observed that m-CPBA alone is capable of converting 2-methylnaphthol (7) into the ortho-quinol 8 and its epoxide in moderate to fair yields.
- 5
- 5aA. A. Zagulyaeva, C. T. Banek, M. S. Yusubov, V. V. Zhdankin, Org. Lett. 2010, 12, 4644;
- 5bH. Hussain, I. R. Green, I. Ahmed, Chem. Rev. 2013, 113, 3329;
- 5cE. A. Merritt, V. M. T. Carneiro, L. F. Silva, Jr., B. Olofsson, J. Org. Chem. 2010, 75, 7416;
- 5dM. Iinuma, K. Moriyama, H. Togo, Synlett 2012, 23, 2663;
- 5eA. K. Mailyan, I. M. Geraskin, V. N. Nemykin, V. V. Zhdankin, J. Org. Chem. 2009, 74, 8444;
- 5fS. Altermann, S. Schäfer, T. Wirth, Tetrahedron 2010, 66, 5902.
- 6A. R. Katritzky, J. K. Gallos, H. Dupont Durst, Magn. Reson. Chem. 1989, 27, 815; Although 13C NMR chemical shifts enable the assignment of the oxidation state of the iodine atom, the structures of iodanes 4–6, for which we could not obtain X-ray quality crystals, remain hypothetical.
- 7CCDC 953640 (B), 983782 ((+)-13 e), 983783 ((−)-13 e), 989094 ((S)-15), 989095 ((R)-2 a), 989096 ((R)-3 a), and 989315 ((S)-1 c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 8The para-quinone 9 is probably derived from 8 through an allylic shift, followed by the oxidation of the resulting para-quinol or the rearomatized para-hydroquinone. Dimer 10 is also a contaminant of commercially available 7, which is highly sensitive to this oxidative dimerization.
- 9
- 9aI. A. Shuklov, N. V. Dubrovina, A. Börner, Synthesis 2007, 2925;
- 9bM. Ito, C. Ogawa, N. Yamaoka, H. Fujioka, T. Dohi, Y. Kita, Molecules 2010, 15, 1918.
- 10
- 10aJ. K. Boppisetti, V. B. Birman, Org. Lett. 2009, 11, 1221;
- 10bS. Dong, J. Zhu, J. A. Porco, Jr., J. Am. Chem. Soc. 2008, 130, 2738;
- 10cS. Quideau, L. Pouységu, D. Deffieux, Synlett 2008, 467;
- 10dJ. Gagnepain, R. Méreau, D. Dejugnac, J.-M. Léger, F. Castet, D. Deffieux, L. Pouységu, S. Quideau, Tetrahedron 2007, 63, 6493;
- 10eN. Lebrasseur, J. Gagnepain, A. Ozanne-Beaudenon, J.-M. Léger, S. Quideau, J. Org. Chem. 2007, 72, 6280;
- 10fJ. Gagnepain, F. Castet, S. Quideau, Angew. Chem. 2007, 119, 1555;
10.1002/ange.200604610 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1533, and J. Gagnepain, F. Castet, S. Quideau, Angew. Chem. 2008, 120, 638;10.1002/ange.200890007 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 628;
- 10gD. Magdziak, A. A. Rodriguez, R. W. Van De Water, T. R. R. Pettus, Org. Lett. 2002, 4, 285.
- 11aR. M. Carman, L. K. Lambert, W. T. Robinson, J. M. A. M. Van Dongen, Aust. J. Chem. 1986, 39, 1843;
- 11bL. Pouységu, S. Chassaing, D. Dejugnac, A.-M. Lamidey, K. Miqueu, J.-M. Sotiropoulos, S. Quideau, Angew. Chem. 2008, 120, 3608;
10.1002/ange.200705816 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3552;
- 11cC. P. Falshaw, A. Franklinos, J. Chem. Soc. Perkin Trans. 1 1984, 95.
- 12
- 12aM. Ochiai, Y. Takaoka, Y. Masaki, Y. Nagao, M. Shiro, J. Am. Chem. Soc. 1990, 112, 5677;
- 12bM. Ochiai, Y. Kitagawa, N. Takayama, Y. Takaoka, M. Shiro, J. Am. Chem. Soc. 1999, 121, 9233;
- 12cC. Röben, J. A. Souto, E. C. Escudero-Adán, K. Muñiz, Org. Lett. 2013, 15, 1008;
- 12dS. Brenet, F. Berthiol, J. Einhorn, Eur. J. Org. Chem. 2013, 8094;
- 12eQ.-H. Deng, J.-C. Wang, Z.-J. Xu, C.-Y. Zhou, C.-M. Che, Synthesis 2011, 2959.