Modulation of Dual Electronic Circuits of [26]Hexaphyrins Using Internal Aromatic Straps†
Hirotaka Mori
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502 (Japan)
Search for more papers by this authorDr. Jong Min Lim
Spectroscopy Laboratory for Functional p -Electronic Systems and Department of Chemistry, Yonsei University, Seoul 120-749 (Korea)
Search for more papers by this authorCorresponding Author
Prof. Dr. Dongho Kim
Spectroscopy Laboratory for Functional p -Electronic Systems and Department of Chemistry, Yonsei University, Seoul 120-749 (Korea)
Dongho Kim, Spectroscopy Laboratory for Functionalp-Electronic Systems and Department of Chemistry, Yonsei University, Seoul 120-749 (Korea)===
Atsuhiro Osuka, Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502 (Japan)===
Search for more papers by this authorCorresponding Author
Prof. Dr. Atsuhiro Osuka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502 (Japan)
Dongho Kim, Spectroscopy Laboratory for Functionalp-Electronic Systems and Department of Chemistry, Yonsei University, Seoul 120-749 (Korea)===
Atsuhiro Osuka, Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502 (Japan)===
Search for more papers by this authorHirotaka Mori
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502 (Japan)
Search for more papers by this authorDr. Jong Min Lim
Spectroscopy Laboratory for Functional p -Electronic Systems and Department of Chemistry, Yonsei University, Seoul 120-749 (Korea)
Search for more papers by this authorCorresponding Author
Prof. Dr. Dongho Kim
Spectroscopy Laboratory for Functional p -Electronic Systems and Department of Chemistry, Yonsei University, Seoul 120-749 (Korea)
Dongho Kim, Spectroscopy Laboratory for Functionalp-Electronic Systems and Department of Chemistry, Yonsei University, Seoul 120-749 (Korea)===
Atsuhiro Osuka, Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502 (Japan)===
Search for more papers by this authorCorresponding Author
Prof. Dr. Atsuhiro Osuka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502 (Japan)
Dongho Kim, Spectroscopy Laboratory for Functionalp-Electronic Systems and Department of Chemistry, Yonsei University, Seoul 120-749 (Korea)===
Atsuhiro Osuka, Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502 (Japan)===
Search for more papers by this authorThe work at Kyoto was supported by Grants-in-Aid from MEXT (grant number 25220802 (S)) for Scientific Research. The work at Yon-sei was supported by the Midcareer Researcher Program of the Ministry of Education, Science, and Technology (MEST) of Korea (grant number 2010-0029668). Support from the Global Research Laboratory (GRL) Program of the Ministry of Education, Science, and Technology (MEST) of Korea (grant number 2013-8-1472) is acknowledged. H.M. acknowledges a JSPS Fellowship for Young Scientists.
Graphical Abstract
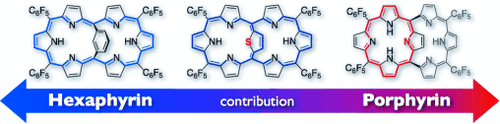
Internal bridges: [26]Hexaphyrins with an aromatic strap in 5,20 position have two potentially cyclic conjugated networks, that is, [18]porphyrin and [26]hexaphyrin (see picture), and show a formal analogy with [18]annuleno[18]annulens. 1,3-Phenylene-, 2,5-thienylene-, and 2,5-pyrrylene-bridged [26]hexaphyrins have been synthesized and characterized.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201308545_sm_miscellaneous_information.pdf6.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Nakagawa, The Chemistry of Annulenes—From the Standpoint of Organic Chemistry—, Osaka University Press, Osaka, 1996;
- 1bM. Nakagawa, Angew. Chem. 1979, 91, 215; Angew. Chem. Int. Ed. Engl. 1979, 18, 202.
- 2
- 2aJ. L. Sessler, D. Seidel, Angew. Chem. 2003, 115, 5292;
10.1002/ange.200200561 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5134;
- 2bH. Furuta, H. Maeda, A. Osuka, Chem. Commun. 2002, 1795;
- 2cT. K. Chandrashekar, A. Venkatraman, Acc. Chem. Res. 2003, 36, 676;
- 2dS. Shimizu, A. Osuka, Eur. J. Inorg. Chem. 2006, 1319;
- 2eJ.-Y. Shin, H. Furuta, K. Yoza, S. Igarashi, A. Osuka, J. Am. Chem. Soc. 2001, 123, 7190;
- 2fM. Stępień, N. Sprutta, L. Latos-Grażyński, Angew. Chem. 2011, 123, 4376;
10.1002/ange.201003353 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4288;
- 2gS. Saito, A. Osuka, Angew. Chem. 2011, 123, 4432; Angew. Chem. Int. Ed. 2011, 50, 4342;
- 2hA. Osuka, S. Saito, Chem. Commun. 2011, 47, 4330.
- 3
- 3aS. Mori, A. Osuka, J. Am. Chem. Soc. 2005, 127, 8030;
- 3bS. Saito, K. Furukawa, A. Osuka, Angew. Chem. 2009, 121, 8230; Angew. Chem. Int. Ed. 2009, 48, 8086;
- 3cY. Kamimura, S. Shimizu, A. Osuka, Chem. Eur. J. 2007, 13, 1620.
- 4
- 4aS. Saito, J.-Y. Shin, J. M. Lim, K. S. Kim, D. Kim, A. Osuka, Angew. Chem. 2008, 120, 9803; Angew. Chem. Int. Ed. 2008, 47, 9657;
- 4bJ. M. Lim, J.-Y. Y. Tanaka, S. Saito, A. Osuka, D. Kim, J. Am. Chem. Soc. 2010, 132, 3105.
- 5aV. G. Anand, S. Saito, S. Shimizu, A. Osuka, Angew. Chem. 2005, 117, 7410;
10.1002/ange.200502769 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7244;
- 5bM. Suzuki, A. Osuka, J. Am. Chem. Soc. 2007, 129, 464.
- 6G. Karthik, M. Sneha, V. P. Raja, J. M. Lim, D. Kim, A. Srinivasan, T. K. Chandrashekar, Chem. Eur. J. 2013, 19, 1886.
- 7One referee wondered about the formation of 1,3-benziporphyrin, thiaporphyrin, or porphyrin in these condensation reactions. But these molecules were not detected or detected in very small amounts.
- 8
- 8aCrystallographic data for 1: triclinic, space group
 (no. 2), a=18.8577(3), b=20.0669(4), c=20.2441(4) Å, α=66.1520(8), β=62.6962(8), γ=78.0242(8), V=6223.7(2) Å3, T=93 K, ρcalcd=1.555 g cm–3, Z=2, R1=0.0779 (I>2σ(I)), Rw=0.2653 (all data), GOF=1.046. CCDC 958400 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif;
(no. 2), a=18.8577(3), b=20.0669(4), c=20.2441(4) Å, α=66.1520(8), β=62.6962(8), γ=78.0242(8), V=6223.7(2) Å3, T=93 K, ρcalcd=1.555 g cm–3, Z=2, R1=0.0779 (I>2σ(I)), Rw=0.2653 (all data), GOF=1.046. CCDC 958400 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif;
- 8bCrystallographic data for 2: monoclinic, space group C2 m (no. 12), a=18.7976(3), b=27.6642(5), c=23.9062(4) Å, β=103.7880(7)°, V=12073.5(4) Å3, T=93 K, ρcalcd=1.496 g cm−3, Z=8, R1=0.0656 (I>2σ(I)), Rw=0.1846 (all data), GOF=1.006. CCDC 958401 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif;
- 8cCrystallographic data for 3: monoclinic, space group P21/a (no. 14), a=17.2023(3), b=34.7013(6), c=17.8053(3) Å, β=110.3636(8)°, V=9964.5(3) Å3, T=93 K, ρcalcd=1.711 g cm−3, Z=4, R1=0.1511 (I>2σ(I)), Rw=0.4395 (all data), GOF=1.099. CCDC 958402 CCDC 6.. contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 9The unit cell contained two different hexaphyrins. Thus, two structural parameters are given for 1–3.
- 10T. Koide, G. Kashiwazaki, M. Suzuki, K. Furukawa, M.-C. Yoon, S. Cho, D. Kim, A. Osuka, Angew. Chem. 2008, 120, 9807;
10.1002/ange.200804570 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9661.
- 11Z. S. Yoon, J. H. Kwon, M.-C. Yoon, M. K. Koh, S. B. Noh, J. L. Sessler, J. T. Lee, D. Seidel, A. Aguilar, S. Shimizu, M. Suzuki, A. Osuka, D. Kim, J. Am. Chem. Soc. 2006, 128, 14128.
- 12Pyrrylene-based three-dimensional or bicyclic systems:
- 12aA. Andrievsky, F. Ahuis, J. L. Sessler, F. Vögtle, D. Gudat, M. Moini, J. Am. Chem. Soc. 1998, 120, 9712;
- 12bC. Bucher, R. S. Zimmerman, V. Lynch, J. L. Sessler, J. Am. Chem. Soc. 2001, 123, 9716.