Thiols and Selenols as Electron-Relay Catalysts for Disulfide-Bond Reduction†
John C. Lukesh III
Department of Chemistry, 1101 University Avenue, University of Wisconsin-Madison, Madison, WI 53706 (USA) http://www.biochem.wisc.edu/faculty/raines/lab
Search for more papers by this authorDr. Brett VanVeller
Department of Chemistry, 1101 University Avenue, University of Wisconsin-Madison, Madison, WI 53706 (USA) http://www.biochem.wisc.edu/faculty/raines/lab
Search for more papers by this authorCorresponding Author
Prof. Ronald T. Raines
Department of Chemistry, 1101 University Avenue, University of Wisconsin-Madison, Madison, WI 53706 (USA) http://www.biochem.wisc.edu/faculty/raines/lab
Department of Biochemistry, 433 Babcock Drive, University of Wisconsin-Madison, Madison, WI 53706 (USA)
Department of Chemistry, 1101 University Avenue, University of Wisconsin-Madison, Madison, WI 53706 (USA) http://www.biochem.wisc.edu/faculty/raines/lab===Search for more papers by this authorJohn C. Lukesh III
Department of Chemistry, 1101 University Avenue, University of Wisconsin-Madison, Madison, WI 53706 (USA) http://www.biochem.wisc.edu/faculty/raines/lab
Search for more papers by this authorDr. Brett VanVeller
Department of Chemistry, 1101 University Avenue, University of Wisconsin-Madison, Madison, WI 53706 (USA) http://www.biochem.wisc.edu/faculty/raines/lab
Search for more papers by this authorCorresponding Author
Prof. Ronald T. Raines
Department of Chemistry, 1101 University Avenue, University of Wisconsin-Madison, Madison, WI 53706 (USA) http://www.biochem.wisc.edu/faculty/raines/lab
Department of Biochemistry, 433 Babcock Drive, University of Wisconsin-Madison, Madison, WI 53706 (USA)
Department of Chemistry, 1101 University Avenue, University of Wisconsin-Madison, Madison, WI 53706 (USA) http://www.biochem.wisc.edu/faculty/raines/lab===Search for more papers by this authorWe are grateful to Prof. H. J. Reich for contributive discussions. B.V. was supported by postdoctoral fellowship 289613 (CIHR). This work was supported by grant R01 GM044783 (NIH). This work made use of the National Magnetic Resonance Facility at Madison, which is supported by grants P41 RR002301 and P41 GM066326 (NIH), and the Biophysics Instrumentation Facility, which was established with grants BIR-9512577 (NSF) and S10 RR13790 (NIH).
Graphical Abstract
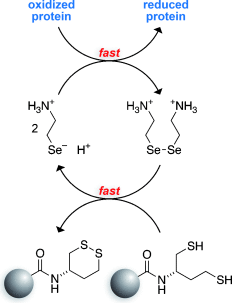
Pass them on! Dithiobutylamine immobilized on a resin is a useful reagent for the reduction of disulfide bonds. Its ability to reduce a disulfide bond in a protein is enhanced greatly if used along with a soluble strained cyclic disulfide or mixed diselenide that relays electrons from the resin to the protein. This electron-relay catalysis system provides distinct advantages over the use of excess soluble reducing agent alone.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201307481_sm_miscellaneous_information.pdf1.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Oxidative Folding of Peptides and Proteins (Ed.: ), The Royal Society of Chemistry, Cambridge, UK, 2009.
- 2
- 2aH. Kadokura, F. Katzen, J. Beckwith, Annu. Rev. Biochem. 2003, 72, 111–135;
- 2bB. P. Tu, J. S. Weissman, J. Cell Biol. 2004, 164, 341–346;
- 2cM. Depuydt, J. Messens, J.-F. Collet, Antioxid. Redox Signaling 2011, 15, 49–66;
- 2dI. Braakman, N. J. Bulleid, Annu. Rev. Biochem. 2011, 80, 71–99.
- 3
- 3aE. A. Kersteen, R. T. Raines, Antioxid. Redox Signaling 2003, 5, 413–424;
- 3bW. J. Lees, Curr. Opin. Chem. Biol. 2008, 12, 740–745;
- 3cS. Yamaguchi, E. Yamamoto, T. Mannen, T. Nagamune, Biotechnol. J. 2013, 8, 17–31.
- 4J. C. Lukesh III, M. J. Palte, R. T. Raines, J. Am. Chem. Soc. 2012, 134, 4057–4059.
- 5
- 5aJ. Houk, G. M. Whitesides, J. Am. Chem. Soc. 1987, 109, 6825–6836;
- 5bJ. A. Burns, G. M. Whitesides, J. Am. Chem. Soc. 1990, 112, 6296–6303;
- 5cW. J. Lees, G. H. Whitesides, J. Org. Chem. 1993, 58, 642–647.
- 6C. C. Leznoff, Acc. Chem. Res. 1978, 11, 327–333.
- 7
- 7aW. Rapp, L. Zhang, E. Bayer in Innovations and Perspectives in Solid-Phase Synthesis (Ed.: ), Mayflower, Birmingham, 1990, pp. 205–210;
- 7bR. Quarrell, T. D. W. Claridge, G. W. Weaver, G. Lowe, Mol. Diversity 1996, 1, 223–232.
- 8
- 8aW. H. Scouten, G. L. Firestone, Biochim. Biophys. Acta Protein Struct. 1976, 453, 277–284;
- 8bR. A. Amos, S. M. Fawcett, J. Org. Chem. 1984, 49, 2637–2639;
- 8cK. J. Woycechowsky, B. A. Hook, R. T. Raines, Biotechnol. Prog. 2008, 19, 1307–1314;
- 8dC. Bienvenu, J. Greiner, P. Vierling, C. Di Giorgio, Tetrahedron Lett. 2010, 51, 3309–3311;
- 8eG. Miralles, P. Verdie, K. Puget, A. Maurras, J. Martinez, G. Subra, ACS Comb. Sci. 2013, 15, 169–173.
- 9
- 9aI. Schechter, A. Berger, Biochem. Biophys. Res. Commun. 1967, 27, 157–162;
- 9bR. W. Pickersgill, G. W. Harris, E. Garman, Acta Crystallogr. Sect. B 1992, 48, 59–67.
- 10G. L. Kenyon, Biochemistry 1975, 14, 766–771.
- 11
- 11aR. Singh, G. M. Whitesides, J. Org. Chem. 1991, 56, 2332–2337;
- 11bW. J. Lees, R. Singh, G. M. Whitesides, J. Org. Chem. 1991, 56, 7328–7331.
- 12
- 12aC. Hwang, A. J. Sinskey, H. F. Lodish, Science 1992, 257, 1496–1502;
- 12bI. Ohtsu, N. Wiriyathanawudhiwong, S. Morigasaki, T. Nakatani, H. Kadokura, H. Takagi, J. Biol. Chem. 2010, 285, 17479–17487;
- 12cK. Van Laer, C. J. Hamilton, J. Messens, Antioxid. Redox Signaling 2013, 18, 1642–1653.
- 13For an electron “relay” through proteins, see: M. Wang, J. Gao, P. Müller, B. Giese, Angew. Chem. 2009, 121, 4296–4298; Angew. Chem. Int. Ed. 2009, 48, 4232–4234.
- 14G. W. Harris, R. W. Pickersgill, B. Howlin, D. S. Moss, Acta Crystallogr. Sect. B 1992, 48 (Pt 1), 67–75.
- 15
- 15aS. M. Bachrach, C. J. Walker, F. Lee, S. Royce, J. Org. Chem. 2007, 72, 5174–5182;
- 15bI. B. Baldus, F. Grater, Biophys. J. 2012, 102, 622–629.
- 16aH. C. Brown, M. Borkowski, J. Am. Chem. Soc. 1952, 74, 1894–1902;
- 16bK. J. Woycechowsky, K. D. Wittrup, R. T. Raines, Chem. Biol. 1999, 6, 871–879.
- 17A. Fava, A. Iliceto, E. Camera, J. Am. Chem. Soc. 1957, 79, 833–838.
- 18R. Singh, G. M. Whitesides, J. Am. Chem. Soc. 1990, 112, 6304–6309.
- 19
- 19aW. H. H. Günther, M. N. Salzman, Ann. N. Y. Acad. Sci. 1972, 192, 25–43;
- 19bG. Mugesh, W. W. du Mont, H. Sies, Chem. Rev. 2001, 101, 2125–2179;
- 19cC. Jacob, G. I. Giles, N. M. Giles, H. Sies, Angew. Chem. 2003, 115, 4890–4907;
10.1002/ange.200300573 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4742–4758;
- 19dV. A. Shchedrina, S. V. Novoselov, M. Y. Malinouski, V. N. Gladyshev, Proc. Natl. Acad. Sci. USA 2007, 104, 13919–13924;
- 19eE. S. J. Arnér, Exp. Cell Res. 2010, 316, 1296–1303;
- 19fE. L. Ruggles, G. W. Snider, R. J. Hondal in Selenium: Its Molecular Biology and Role in Human Health, 3rd ed. ), Springer, New York, 2011, pp. 73–84;
- 19gR. J. Hondal, E. L. Ruggles, Amino Acids 2011, 41, 73–89;
- 19hN. Metanis, J. Beld, D. Hilvert in Patai’s Chemistry of Functional Groups, Wiley, Hoboken, 2011, DOI: ;
- 19iT. Nauser, D. Steinmann, W. H. Koppenol, Amino Acids 2012, 42, 39–44;
- 19jR. J. Hondal, S. M. Marino, V. N. Gladyshev, Antioxid. Redox Signaling 2013, 18, 1675–1689.
- 20
- 20aR. Singh, G. M. Whitesides, J. Org. Chem. 1991, 56, 6931–6933;
- 20bR. Singh, L. Kats, Anal. Biochem. 1995, 232, 86–91;
- 20cD. Steinmann, T. Nauser, W. H. Koppenol, J. Org. Chem. 2010, 75, 6696–6699.
- 21
- 21aY. Chen, W. Maret, Eur. J. Biochem. 2001, 268, 3346–3353;
- 21bR. Singh, E. K. Maloney, Anal. Biochem. 2002, 304, 147–156;
- 21cJ. Beld, K. J. Woycechowsky, D. Hilvert, Biochemistry 2008, 47, 6985–6987;
- 21dA. Walewska, M. M. Zhang, J. J. Skalicky, D. Yoshikami, B. M. Olivera, G. Bulaj, Angew. Chem. 2009, 121, 2255–2258;
10.1002/ange.200806085 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 2221–2224;
- 21eJ. Beld, K. J. Woycechowsky, D. Hilvert, Biochemistry 2009, 48, 4662–4662;
- 21fJ. Beld, K. J. Woycechowsky, D. Hilvert, J. Biotechnol. 2010, 150, 481–489;
- 21gA. M. Steiner, K. J. Woycechowsky, B. M. Olivera, G. Bulaj, Angew. Chem. 2012, 124, 5678–5682;
10.1002/ange.201200062 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5580–5584;
- 21hN. Metanis, D. Hilvert, Angew. Chem. 2012, 124, 5683–5686; Angew. Chem. Int. Ed. 2012, 51, 5585–5588.
- 22M. Iwaoka, T. Takahashi, S. Tomoda, Heteroat. Chem. 2001, 12, 293–299.
- 23
- 23aD. E. Shafer, J. K. Inman, A. Lees, Anal. Biochem. 2000, 282, 161–164;
- 23b The Protein Protocols Handbook (Ed.: ), Humana, Totowa, NJ, 2002.
- 24G. T. Hermanson, Bioconjugate Techniques, 3rd ed., Academic Press, New York, NY, 2013.