Formal Ring-Opening/Cross-Coupling Reactions of 2-Pyrones: Iron-Catalyzed Entry into Stereodefined Dienyl Carboxylates†
Dr. Chang-Liang Sun
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
Search for more papers by this authorCorresponding Author
Prof. Alois Fürstner
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)===Search for more papers by this authorDr. Chang-Liang Sun
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
Search for more papers by this authorCorresponding Author
Prof. Alois Fürstner
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)===Search for more papers by this authorGenerous financial support by the MPG, the Alexander-von-Humboldt Foundation (fellowship to C.-L.S.), and the Fonds der Chemischen Industrie is gratefully acknowledged. We thank the analytical departments of our Institute for excellent support.
Graphical Abstract
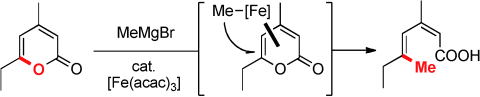
Open access: Despite the exceptional level of sophistication in cross-coupling chemistry, reactions of substrates that incorporate the leaving group as an integral part into a heterocyclic scaffold are scarce. The title reaction outlines the utility of this reaction format (see scheme; acac=acetylacetonate), provides a convenient entry into stereodefined diene carboxylates, and adds a new chapter to the field of iron catalysis.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201307028_sm_miscellaneous_information.pdf2.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. D. Sherry, A. Fürstner, Acc. Chem. Res. 2008, 41, 1500–1511.
- 2E. Nakamura, N. Yoshikai, J. Org. Chem. 2010, 75, 6061–6067.
- 3T. Hatakeyama, K. Ishizuka, M. Nakamura, J. Synth. Org. Chem. Jpn. 2011, 69, 1282–1298.
- 4G. Cahiez, H. Avedissian, Synthesis 1998, 1199–1205.
- 5E. B. Bauer, Curr. Org. Chem. 2008, 12, 1341–1369.
- 6W. M. Czaplik, M. Mayer, J. Cvengroš, A. Jacobi von Wangelin, ChemSusChem 2009, 2, 396–417.
- 7K. Gopalaiah, Chem. Rev. 2013, 113, 3248–3296.
- 8A. Fürstner, R. Martin, Chem. Lett. 2005, 34, 624–629.
- 9
- 9aC. Risatti, K. J. Natalie, Jr., Z. Shi, D. A. Conlon, Org. Process Res. Dev. 2013, 17, 257–264;
- 9bA. Fürstner, A. Leitner, G. Seidel, Org. Synth. 2005, 81, 33–41.
- 10
- 10aA. Fürstner, A. Leitner, M. Méndez, H. Krause, J. Am. Chem. Soc. 2002, 124, 13856–13863;
- 10bA. Fürstner, A. Leitner, Angew. Chem. 2002, 114, 632–635; Angew. Chem. Int. Ed. 2002, 41, 609–612.
- 11
- 11aA. Fürstner, A. Leitner, Angew. Chem. 2003, 115, 320–323;
10.1002/ange.200390071 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 308–311;
- 11bB. Scheiper, F. Glorius, A. Leitner, A. Fürstner, Proc. Natl. Acad. Sci. USA 2004, 101, 11960–11965.
- 12
- 12aB. Scheiper, M. Bonnekessel, H. Krause, A. Fürstner, J. Org. Chem. 2004, 69, 3943–3949;
- 12bG. Seidel, D. Laurich, A. Fürstner, J. Org. Chem. 2004, 69, 3950–3952;
- 12cA. Fürstner, P. Hannen, Chem. Eur. J. 2006, 12, 3006–3019;
- 12dA. Fürstner, A. Schlecker, Chem. Eur. J. 2008, 14, 9181–9191.
- 13For representative studies, see:
- 13aA. L. Silberstein, S. D. Ramgren, N. K. Garg, Org. Lett. 2012, 14, 3796–3799;
- 13bT. Mesganaw, N. K. Garg, Org. Process Res. Dev. 2013, 17, 29–39;
- 13cT. Agarwal, S. P. Cook, Org. Lett. 2013, 15, 96–99;
- 13dO. M. Kuzmina, A. K. Steib, D. Flubacher, P. Knochel, Org. Lett. 2012, 14, 4818–4821;
- 13eM. C. Perry, A. N. Gillett, T. C. Law, Tetrahedron Lett. 2012, 53, 4436–4439;
- 13fG. Cahiez, O. Gager, J. Buendia, C. Patinote, Chem. Eur. J. 2012, 18, 5860–5863;
- 13gR. K. Shiroodi, A. S. Dudnik, V. Gevorgyan, J. Am. Chem. Soc. 2012, 134, 6928–6931;
- 13hS. Gülak, A. Jacobi von Wangelin, Angew. Chem. 2012, 124, 1386–1390;
10.1002/ange.201106110 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1357–1361;
- 13iH. Nishikado, H. Nakatsuji, K. Ueno, R. Nagase, Y. Tanabe, Synlett 2010, 2087–2092;
- 13jC. M. Rao Volla, P. Vogel, Angew. Chem. 2008, 120, 1325–1327;
10.1002/ange.200704858 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1305–1307;
- 13kB.-J. Li, L. Xu, Z.-H. Wu, B.-T. Guan, C.-L. Sun, B.-Q. Wang, Z.-J. Shi, J. Am. Chem. Soc. 2009, 131, 14656–14657;
- 13lU. S. Larsen, L. Martiny, M. Begtrup, Tetrahedron Lett. 2005, 46, 4261–4263;
- 13mK. Itami, S. Higashi, M. Mineno, J. Yoshida, Org. Lett. 2005, 7, 1219–1222;
- 13nN. Hayashi, M. Nakada, Tetrahedron Lett. 2009, 50, 232–235;
- 13oG. Cahiez, O. Gager, V. Habiak, Synthesis 2008, 2636–2644;
- 13pG. Cahiez, V. Habiak, O. Gager, Org. Lett. 2008, 10, 2389–2392.
- 14C.-L. Sun, B.-J. Li, Z.-J. Shi, Chem. Rev. 2011, 111, 1293–1314.
- 15
- 15aFor nickel-catalyzed ring-opening/cross-coupling of cyclic enol ethers, see: E. Wenkert, E. L. Michelotti, C. S. Swindell, M. Tingoli, J. Org. Chem. 1984, 49, 4894–4899;
- 15bP. J. Kocieński, M. Pritchard, S. N. Wadman, R. J. Whitby, C. L. Yeates, J. Chem. Soc. Perkin Trans. 1 1992, 3419–3429;
- 15cP. A. Ashworth, N. J. Dixon, P. J. Kocieński, S. N. Wadman, J. Chem. Soc. Perkin Trans. 1 1992, 3431–3438;
- 15dfor cross-coupling/desulfurization of various sulfur-containing heterocycles, see: T.-Y. Luh, Z.-J. Ni, Synthesis 1990, 89–103;
- 15eM. Tiecco, M. Tingoli, E. Wenkert, J. Org. Chem. 1985, 50, 3828–3831;
- 15fA. Oviedo, A. Arévalo, M. Flores-Alamo, J. J. Garcia, Organometallics 2012, 31, 4039–4045;
- 15gY.-H. Cho, A. Kina, T. Shimada, T. Hayashi, J. Org. Chem. 2004, 69, 3811–3823;
- 15hfor a ring opening Kumada coupling of cyclic ethers, see: J. W. Dankwardt, Angew. Chem. 2004, 116, 2482–2486;
10.1002/ange.200453765 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2428–2432;
- 15ifor formal ring-opening/cross-coupling of cyclic enol ethers by a 1,2-metallate rearrangement, see: K. Jarowicki, P. J. Kocienski, L. Qun, Org. Synth. 2002, 79, 11–18.
- 16For representative studies on uncatalyzed ring-opening of pyrylium and pyridinium salts or dihydrofurans on treatment with organometallic reagents, see the following and literature cited therein:
- 16aM. Furber, J. M. Herbert, R. J. K. Taylor, J. Chem. Soc. Perkin Trans. 1 1989, 683–690;
- 16bB. Feith, H.-M. Weber, G. Maas, Chem. Ber. 1986, 119, 3276–3296;
- 16cT. Fujisawa, Y. Kurita, M. Kawashima, T. Sato, Chem. Lett. 1982, 1641–1642;
- 16dfor accounts on heterocycle ring-opening by other nucleophiles, see: C. D. Vanderwal, J. Org. Chem. 2011, 76, 9555–9567;
- 16eG. Piancatelli, M. D’Auria, F. D’Onofrio, Synthesis 1994, 867–889.
- 17[Fe(acac)3] (99.9+ % purity) is the pre-catalyst of choice because it is cheap, non-hygroscopic, and commercially available. While [(tmeda)FeI2] is similarly effective, the use of [(depe)2FeCl2] or [(dppp)FeCl2] basically halted the conversion (<10 %, GC). No reaction was observed in the absence of an iron salt. depe=bis(diethylphosphino)-ethane; dppp=bis(diphenylphosphino)propane; tmeda=N,N,N′,N′-tetramethylethylenediamine.
- 18It is unlikely that trace impurities of copper in [Fe(acac)3] are the actual catalyst because copper leads to 1,4-addition of Grignard reagents to 2-pyrones without ring opening, see: B. Mao, M. Fañanás-Mastral, B. L. Feringa, Org. Lett. 2013, 15, 286–289.
- 19F. Frébault, M. T. Oliveira, E. Wöstefeld, N. Maulide, J. Org. Chem. 2010, 75, 7962–7965.
- 20T. L. Gilchrist, Heterocyclenchemie, VCH, Weinheim, 1995.
- 21For iron-catalyzed CC bond formation under opening of a strained carbocyclic ring, see: B. D. Sherry, A. Fürstner, Chem. Commun. 2009, 7116–7118.
- 22For a different entry into these motifs starting from pyrones, see: C. Souris, F. Frébault, A. Patel, D. Audisio, K. N. Houk, N. Maulide, Org. Lett. 2013, 15, 3242–3245.
- 23A. Fürstner, K. Majima, R. Martín, H. Krause, E. Kattnig, R. Goddard, C. W. Lehmann, J. Am. Chem. Soc. 2008, 130, 1992–2004.
- 24The ready preparation of the substrates used herein illustrates this aspect. For details, see the Supporting Information. For a particularly convenient new entry into 4-hydroxy-2-pyrones, see: W. Chaładaj, M. Corbet, A. Fürstner, Angew. Chem. 2012, 124, 7035–7039;
10.1002/ange.201203180 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6929–6933.
- 25
- 25aP. T. Northcote, J. W. Blunt, M. H. G. Munro, Tetrahedron Lett. 1991, 32, 6411–6414;
- 25bD. Romo, R. M. Rzasa, H. A. Shea, K. Park, J. M. Langenhan, L. Sun, A. Akhiezer, J. O. Liu, J. Am. Chem. Soc. 1998, 120, 12237–12254;
- 25cG. Pattenden, D. J. Critcher, M. Remuiñán, Can. J. Chem. 2004, 82, 353–365.
- 26W.-K. Low, Y. Dang, T. Schneider-Poetsch, Z. Shi, N. S. Choi, R. M. Rzasa, H. A. Shea, S. Li, K. Park, G. Ma, D. Romo, J. O. Liu, Methods Enzymol. 2007, 431, 303–324.
- 27S. Di Marco, A. Cammas, X. J. Lian, E. N. Kovacs, J. F. Ma, D. T. Hall, R. Mazroui, J. Richardson, J. Pelletier, I. E. Gallouzi, Nat. Commun. 2012, 3, 896.
- 28F. Reyes, R. Martín, R. Fernández, J. Nat. Prod. 2006, 69, 668–670.
- 29M. F. Semmelhack in Organometallics in Synthesis. A Manual, 2nd ed. ), Wiley, Chichester, pp. 1003–1121.
- 30
- 30aK. Fukuhara, H. Urabe, Tetrahedron Lett. 2005, 46, 603–606;
- 30bS. Okada, K. Arayama, R. Murayama, T. Ishizuka, K. Hara, N. Hirone, T. Hata, H. Urabe, Angew. Chem. 2008, 120, 6966–6970; Angew. Chem. Int. Ed. 2008, 47, 6860–6864;
- 30cT. Hata, T. Nakada, Y. T. Oh, N. Hirone, H. Urabe, Adv. Synth. Catal. 2013, 355, 1736–1740.
- 31C. H. DePuy, R. L. Parton, T. Jones, J. Am. Chem. Soc. 1977, 99, 4070–4075.
- 32A. Fürstner, H. Krause, C. W. Lehmann, Angew. Chem. 2006, 118, 454–458;
10.1002/ange.200502859 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 440–444.
- 33A. Fürstner, R. Martin, H. Krause, G. Seidel, R. Goddard, C. W. Lehmann, J. Am. Chem. Soc. 2008, 130, 8773–8787.
- 34For a leading reference see the following and the literature cited therein: B. Schmidt, O. Kunz, Eur. J. Org. Chem. 2012, 1008–1018.
- 35
- 35aFor structural evidence for C-metalated iron ester enolates, see: P. Laurent, S. Sabo-Etienne, A.-M. Larsonneur, H. des Abbayes, J. Chem. Soc. Chem. Commun. 1988, 929–930;
- 35bfor O-metalated iron ketone enolates, see: S. Sabo-Etienne, H. des Abbayes, L. Toupet, Organometallics 1987, 6, 2262–2263;
- 35cD. Noda, Y. Sunada, T. Hatakeyama, M. Nakamura, H. Nagashima, Chem. Commun. 2012, 48, 12231–12333;
- 35dS. Kiyota, J.-I. Tamuki, N. Komine, M. Hirano, S. Komiya, Chem. Lett. 2005, 34, 498–499.