Multiple Reduction of 2,5-Bis(borolyl)thiophene: Isolation of a Negative Bipolaron by Comproportionation†
Corresponding Author
Prof. Dr. Holger Braunschweig
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)===Search for more papers by this authorProf. Dr. Vladimir Dyakonov
Fakultät für Physik und Astronomie, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Bernd Engels
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)===Search for more papers by this authorZarah Falk
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorChristian Hörl
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorJohannes H. Klein
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorThomas Kramer
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorHannes Kraus
Fakultät für Physik und Astronomie, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg (Germany)
Search for more papers by this authorDr. Ivo Krummenacher
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorProf. Dr. Christoph Lambert
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorChristof Walter
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Holger Braunschweig
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)===Search for more papers by this authorProf. Dr. Vladimir Dyakonov
Fakultät für Physik und Astronomie, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Bernd Engels
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)===Search for more papers by this authorZarah Falk
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorChristian Hörl
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorJohannes H. Klein
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorThomas Kramer
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorHannes Kraus
Fakultät für Physik und Astronomie, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg (Germany)
Search for more papers by this authorDr. Ivo Krummenacher
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorProf. Dr. Christoph Lambert
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorChristof Walter
Institut für Chemie und Pharmazie, Julius-Maximilians-Universität Würzburg (Germany)
Search for more papers by this authorThis study was performed within the framework of the Graduate College GRK 1221. C.W. thanks the Fonds der Chemischen Industrie for a fellowship. We thank Dr. Charles Gould for magnetic measurements.
Graphical Abstract
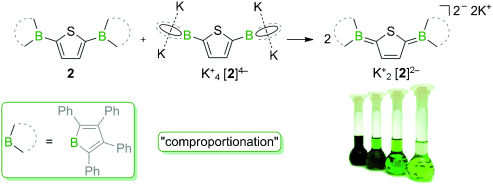
The 2,5-bis(borolyl)thiophene 2, a conjugated acceptor–π–acceptor system, can be reduced to the monoradical anion [2].−, the dianion [2]2−, and the tetraanion [2]4−. The dianion [2]2− was also prepared by a comproportionation reaction (see scheme) and features an absorption maximum in the near-IR region (λmax=800 nm), which is characteristic of a bipolaron with a quinoidal structure.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201306969_sm_miscellaneous_information.pdf1.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Mishra, P. Bäuerle, Angew. Chem. 2012, 124, 2060–2109; Angew. Chem. Int. Ed. 2012, 51, 2020–2067;
- 1bA. Mishra, C.-Q. Ma, P. Bäuerle, Chem. Rev. 2009, 109, 1141–1276;
- 1cK. Müllen, G. Wegner, Electronic Materials: The Oligomer Approach, Wiley-VCH, Weinheim, 1998;
10.1002/9783527603220 Google Scholar
- 1dD. Fichou, Handbook of Oligo- and Polythiophenes, Wiley-VCH, Weinheim, 1999;
- 1eY.-J. Cheng, S.-H. Yang, C.-S. Hsu, Chem. Rev. 2009, 109, 5868–5923.
- 2
- 2aM. Garcia, L. Fomina, S. Fomine, Synth. Met. 2010, 160, 2515–2519;
- 2bJ. Casado, R. P. Ortiz, J. T. L. Navarete, Chem. Soc. Rev. 2012, 41, 5672–5686;
- 2cZ. B. Henson, K. Müllen, G. C. Bazan, Nat. Chem. 2012, 4, 699–704.
- 3
- 3aA. R. Bishop, D. K. Campell, K. Fesser, Mol. Cryst. Liq. Cryst. 1981, 77, 253–264;
- 3bJ. L. Brédas, R. R. Chance, R. Silbey, Mol. Cryst. Liq. Cryst. 1981, 77, 319–332;
- 3cL. W. Shacklette, J. F. Wolf, S. Gould, R. H. Baughman, J. Chem. Phys. 1988, 88, 3955–3961;
- 3dP. Bäuerle, U. Segelbacher, A. Maier, M. Mehring, J. Am. Chem. Soc. 1993, 115, 10217–10223;
- 3eY. Shimoi, S. Abe, Phys. Rev. B 1994, 50, 14781;
- 3fA. J. W. Tol, Synth. Met. 1995, 74, 95–98;
- 3gW. Kaim, S. Zališ, Inorg. Chem. 1996, 35, 3039–3042;
- 3hA. J. W. Tol, Chem. Phys. 1996, 208, 73–79;
- 3iY. Furukawa, J. Phys. Chem. 1996, 100, 15644–15653;
- 3jA. J. Heeger, S. Kivelson, J. R. Schrieffer, W. P. Su, Mod. Phys. 1988, 60, 781;
- 3kJ. van Haare, E. E. Havinga, J. L. J. van Dongen, R. A. J. Janssen, J. Cornil, J. L. Brédas, Chem. Eur. J. 1998, 4, 1509–1522;
10.1002/(SICI)1521-3765(19980807)4:8<1509::AID-CHEM1509>3.0.CO;2-# CAS Web of Science® Google Scholar
- 3lC. Lambert, G. Nöll, J. Am. Chem. Soc. 1999, 121, 8434–8442;
- 3mY. Gao, C.-G. Liu, Y.-S. Jiang, J. Phys. Chem. A 2002, 106, 5380–5384;
- 3nM. Kozaki, Y. Yonezawa, K. Okada, Org. Lett. 2002, 4, 4535–4538;
- 3oJ. L. Brédas, V. M. Geskin, ChemPhysChem 2003, 4, 498;
- 3pT. Nishinaga, A. Wakamiya, D. Yamazaki, K. Komatsu, J. Am. Chem. Soc. 2004, 126, 3163–3174;
- 3qS. Zheng, S. Barlow, C. Risko, T. L. Kinnibrugh, V. N. Khrustalev, S. C. Jones, M. Y. Antipin, N. M. Tucker, T. V. Timofeeva, V. Coropceanu, J.-L. Brédas, S. R. Marder, J. Am. Chem. Soc. 2006, 128, 1812–1827;
- 3rS. Fratiloiu, F. C. Grozema, Y. Koizumi, S. Seki, A. Saeki, S. Tagawa, S. P. Dudek, L. D. A. Siebbeles, J. Phys. Chem. B 2006, 110, 5984–5993;
- 3sT. J. Savenije, A. Sperlich, H. Kraus, O. Poluektov, M. Heeney, V. Dyakonov, Phys. Chem. Chem. Phys. 2011, 13, 16579.
- 4
- 4aW. Kaim, A. Schulz, Angew. Chem. 1984, 96, 611–612; Angew. Chem. Int. Ed. Engl. 1984, 23, 615–616;
- 4bP. P. Power, M. M. Olmstead, J. Am. Chem. Soc. 1986, 108, 4235–4236;
- 4cW. Kaim, A. Schulz, Chem. Ber. 1989, 122, 1863–1868;
- 4dA. Lichtblau, W. Kaim, A. Schulz, T. Stahl, J. Chem. Soc. Perkin Trans. 2 1992, 2, 1497–1501;
- 4eA. Rajca, S. Rajca, S. R. Desai, J. Chem. Soc. Chem. Commun. 1995, 1957–1958;
- 4fF. P. Gabbaï, J. D. Hoefelmeyer, J. Am. Chem. Soc. 2000, 122, 9054–9055;
- 4gW. Kaim, N. S. Hosmane, S. Zalis, J. A. Maguire, W. N. Lipscomb, Angew. Chem. 2009, 121, 5184–5193;
10.1002/ange.200803493 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 5082–5091;
- 4hH. Braunschweig, V. Dyakonov, J. O. C. Jimenez-Halla, K. Kraft, I. Krummenacher, K. Radacki, A. Sperlich, J. Wahler, Angew. Chem. 2012, 124, 3031–3034; Angew. Chem. Int. Ed. 2012, 51, 2977–2980.
- 5
- 5aZ. Yuan, N. J. Taylor, R. Ramachandran, T. B. Marder, Appl. Organomet. Chem. 1996, 10, 305–316;
- 5bT. Noda, Y. Shirota, J. Am. Chem. Soc. 1998, 120, 9714–9715;
- 5cT. Noda, H. Ogawa, Y. Shirota, Adv. Mater. 1999, 11, 283;
- 5dZ. Yuan, J. C. Collings, N. J. Taylor, T. B. Marder, C. Jardin, J.-F. Halet, J. Solid State Chem. 2000, 154, 5–12.
- 6H. Braunschweig, I. Fernández, G. Frenking, T. Kupfer, Angew. Chem. 2008, 120, 1977–1980; Angew. Chem. Int. Ed. 2008, 47, 1951–1954.
- 7H. Braunschweig, A. Damme, J. O. C. Jimenez-Halla, C. Hörl, I. Krummenacher, T. Kupfer, L. Mailänder, K. Radacki, J. Am. Chem. Soc. 2012, 134, 20169–20177.
- 8H. Braunschweig, T. Kupfer, Chem. Commun. 2011, 47, 10903–10914.
- 9
- 9aL. Weber, J. Kahlert, H.-G. Stammler, B. Neumann, Z. Anorg. Allg. Chem. 2008, 634, 1729–1734;
- 9bL. Weber, V. Werner, M. A. Fox, T. B. Marder, S. Schwedler, A. Brockhinke, H.-G. Stammler, B. Neumann, Dalton Trans. 2009, 1339–1351.
- 10F. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, R. Taylor, J. Chem. Soc. Perkin Trans. 2 1987, S 1–S19.
- 11The irreversibility of the redox waves is likely to be due to the fast decomposition of the electrochemically generated anions in the media and the dissociation of THF in this process. The cyclic voltammogram is not improved in CH2Cl2 solution or by using a supporting electrolyte containing [B(C6H3(CF3)2)4]− as the counterion.
- 12H. Braunschweig, F. Breher, C.-W. Chiu, D. Gamon, D. Nied, K. Radacki, Angew. Chem. 2010, 122, 9159–9162; Angew. Chem. Int. Ed. 2010, 49, 8975–8978.
- 13
- 13aT. M. Pappenfus, J. D. Raff, J. Hukkanen, J. R. Burney, J. Casado, S. M. Drew, L. L. Miller, K. R. Mann, J. Org. Chem. 2002, 67, 6015–6024;
- 13bF. Murakami, S. Sasaki, M. Yoshifuji, Angew. Chem. 2002, 114, 2686–2688;
Angew. Chem. Int. Ed. 2002, 41, 2574–2576;
10.1002/1521-3773(20020715)41:14<2574::AID-ANIE2574>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 13cJ. Casado, T. M. Pappenfus, K. R. Mann, E. Orti, P. M. Viruela, B. Milián, V. Hernández, J. T. L. Navarreye, ChemPhysChem 2004, 5, 529.
- 14
- 14aH. Braunschweig, C. W. Chiu, A. Damme, B. Engels, D. Gamon, C. Hörl, T. Kupfer, I. Krummenacher, K. Radacki, C. Walter, Chem. Eur. J. 2012, 18, 14292–14304;
- 14bC.-W. So, D. Watanabe, A. Wakamiya, S. Yamaguchi, Organometallics 2008, 27, 3496–3501.
- 15E. M. Schubert, J. Chem. Educ. 1992, 69, 62.
- 16
- 16aB. O. Roos, P. R. Taylor, P. E. M. Siegbahn, Chem. Phys. 1980, 48, 157;
- 16bB. O. Roos, J. Quantum Chem. 1980, S14, 175.
- 17M. Kleinschmidt, J. Tatchen, C. M. Marian, J. Comput. Chem. 2002, 23, 824–833.
- 18CCDC 937544 (2) and CCDC 937545 ([CoCp*2]2[2]) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.