Copper(I)-Catalyzed Cycloaddition of Bismuth(III) Acetylides with Organic Azides: Synthesis of Stable Triazole Anion Equivalents†
Brady T. Worrell
Department of Chemistry, The Scripps Research Institute, La Jolla, CA 92037 (USA) http://www.scripps.edu/fokin
Search for more papers by this authorDr. Shelby P. Ellery
Department of Chemistry, The Scripps Research Institute, La Jolla, CA 92037 (USA) http://www.scripps.edu/fokin
Search for more papers by this authorCorresponding Author
Prof. Valery V. Fokin
Department of Chemistry, The Scripps Research Institute, La Jolla, CA 92037 (USA) http://www.scripps.edu/fokin
Department of Chemistry, The Scripps Research Institute, La Jolla, CA 92037 (USA) http://www.scripps.edu/fokin===Search for more papers by this authorBrady T. Worrell
Department of Chemistry, The Scripps Research Institute, La Jolla, CA 92037 (USA) http://www.scripps.edu/fokin
Search for more papers by this authorDr. Shelby P. Ellery
Department of Chemistry, The Scripps Research Institute, La Jolla, CA 92037 (USA) http://www.scripps.edu/fokin
Search for more papers by this authorCorresponding Author
Prof. Valery V. Fokin
Department of Chemistry, The Scripps Research Institute, La Jolla, CA 92037 (USA) http://www.scripps.edu/fokin
Department of Chemistry, The Scripps Research Institute, La Jolla, CA 92037 (USA) http://www.scripps.edu/fokin===Search for more papers by this authorThis work was supported by the National Institute of General Medical Sciences, National Institutes of Health (GM-087620). Prof. Jason Hein (U.C. Merced), Dr. David Sarlah, and Will Gutekunst are gratefully acknowledged for helpful scientific discussions.
Graphical Abstract
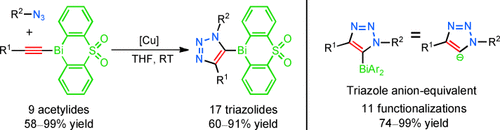
Fully loaded: Readily accessible and shelf-stable 1-bismuth(III) acetylides react rapidly and regiospecifically with organic azides in the presence of a copper(I) catalyst (see scheme). The reaction tolerates many functional groups and gives excellent yields of the previously unreported 5-bismuth triazolides. This uniquely reactive intermediate is functionalized under mild reaction conditions to give fully substituted 1,2,3-triazoles.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201306192_sm_miscellaneous_information.pdf3.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aV. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708;
10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2596;10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 1bC. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057.
- 2
- 2aP. Wu, V. V. Fokin, Aldrichimica Acta 2007, 40, 7–17;
- 2bM. Meldal, C. W. Tornøe, Chem. Rev. 2008, 108, 2952.
- 3
- 3aH. C. Kolb, K. B. Sharpless, Drug Discovery Today 2003, 8, 1128;
- 3bM. Whiting, J. C. Tripp, Y.-C. Lin, W. Lindstrom, A. J. Olson, J. H. Elder, K. B. Sharpless, V. V. Fokin, J. Med. Chem. 2006, 49, 7697;
- 3cB. L. Wilkinson, L. F. Bornaghi, T. A. Houston, S.-A. Poulsen, Drug Design Research Perspectives, Vol. 57 (Ed.: ), Nova, Hauppauge, 2007.
- 4
- 4aA. J. Link, D. A. Tirrell, J. Am. Chem. Soc. 2003, 125, 11164;
- 4bQ. Wang, T. R. Chan, R. Hilgraf, V. V. Fokin, K. B. Sharpless, M. G. Finn, J. Am. Chem. Soc. 2003, 125, 3192;
- 4cJ.-F. Lutz, Z. Zarafshani, Adv. Drug Delivery Rev. 2008, 60, 958.
- 5C. J. Hawker, V. V. Fokin, M. G. Finn, K. B. Sharpless, Aust. J. Chem. 2007, 60, 381.
- 6
- 6aR. A. Evans, Aust. J. Chem. 2007, 60, 384;
- 6bJ. A. Johnson, J. T. Koberstein, M. G. Finn, N. J. Turro, Macromol. Rapid Commun. 2008, 29, 1052.
- 7
- 7aJ. E. Hein, J. C. Tripp, L. B. Krasnova, K. B. Sharpless, V. V. Fokin, Angew. Chem. 2009, 121, 8162;
10.1002/ange.200903558 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8018;
- 7bC. Spiteri, J. E. Moses, Angew. Chem. 2010, 122, 33;
10.1002/ange.200905322 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 31.
- 8Intermolecular couplings:
- 8aJ. Deng, Y.-M. Wu, Q.-Y. Chen, Synlett 2005, 2730; Intramolecular couplings:
- 8bJ. Panteleev, K. Geyer, A. Aguilar-Aguilar, L. Wang, M. Lautens, Org. Lett. 2010, 12, 5092;
- 8cJ. M. Schulman, A. A. Friedman, J. Panteleev, M. Lautens, Chem. Commun. 2012, 48, 55.
- 9
- 9aB. H. M. Kuijpers, G. C. T. Dijkmans, S. Groothuys, P. J. L. M. Quaedflieg, R. H. Blaauw, F. L. van Delft, F. P. J. T. Rutjes, Synlett 2005, 3059;
- 9bD. V. Partyka, L. Gao, T. S. Teets, J. B. Updegraff, N. Deligonul, T. G. Gray, Organometallics 2009, 28, 6171;
- 9cY. Zhou, T. Lecourt, L. Micouin, Angew. Chem. 2010, 122, 2661; Angew. Chem. Int. Ed. 2010, 49, 2607.
- 10A SciFinder search reveals only 20 reactions involving bismuth(III) acetylides, predominately as substrates in structural studies.
- 11For selected reviews on the subject, see:
- 11aL. D. Freedman, G. O. Doak, Chem. Rev. 1982, 82, 15;
- 11bJ.-P. Finet, Chem. Rev. 1989, 89, 1487;
- 11cH. Suzuki, T. Ikegami, Y. Matano, Synthesis 1997, 249;
- 11dG. I. Elliott, J. P. Konopelski, Tetrahedron 2001, 57, 5683;
- 11e Organobismuth Chemistry (Eds.: ), Elsevier, Amsterdam, 2001;
- 11fJ. Luan, L. Zhang, Z. Hu, Molecules 2011, 16, 4191.
- 12Untethered bismuth(III) acetylide complexes:
- 12aH. Hartmann, G. Habenicht, W. Reiss, Z. Anorg. Allg. Chem. 1962, 317, 54;
- 12bK. Rudolph, M. Wieber, Z. Naturforsch. B 1991, 46, 1319;
- 12cY. Matano, M. Kinoshita, H. Suzuki, Bull. Chem. Soc. Jpn. 1992, 65, 3504; Tethered bismuth(III) acetylide complexes:
- 12dH. Suzuki, T. Murafuji, N. Azuma, J. Chem. Soc. Perkin Trans. 1 1992, 1593;
- 12eS. Shimada, O. Yamazaki, T. Tanaka, Y. Suzuki, M. Tanaka, J. Organomet. Chem. 2004, 689, 3012.
- 13K. Ohkata, S. Takemoto, M. Ohnishi, K.-y. Akiba, Tetrahedron Lett. 1989, 30, 4841.
- 14T. Ogawa, T. Ikegami, T. Hikasa, N. Ono, H. Suzuki, J. Chem. Soc. Perkin Trans. 1 1994, 3479.
- 15S. Oae, Y. Uchida, Acc. Chem. Res. 1991, 24, 202.
- 16Palladium(0)-catalyzed reaction of triaryl bismuth(III) complexes and acid chlorides:
- 16aD. H. R. Barton, N. Ozbalik, M. Ramesh, Tetrahedron 1988, 44, 5661. Interestingly, when oxalyl chloride was reacted with a triaryl bismuth(III) complex with a Pd0 catalyst, diaryl ketones, rather than 1,2-diketone products were formed:
- 16bM. L. N. Rao, V. Venkatesh, P. Dasgupta, Tetrahedron Lett. 2010, 51, 4975.
- 17H. Yamamoto, N. Momiyama, Chem. Commun. 2005, 3514–3525.
- 18B. T. Worrell, J. A. Malik, V. V. Fokin, Science 2013, 340, 457–460.