Chiral Phosphoric Acid Directed Regioselective Acetalization of Carbohydrate-Derived 1,2-Diols†
Dr. Enoch Mensah
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA)
Search for more papers by this authorNicole Camasso
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA)
Search for more papers by this authorWill Kaplan
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Pavel Nagorny
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA)
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA)===Search for more papers by this authorDr. Enoch Mensah
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA)
Search for more papers by this authorNicole Camasso
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA)
Search for more papers by this authorWill Kaplan
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Pavel Nagorny
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA)
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA)===Search for more papers by this authorWe are grateful to the University of Michigan for financial support.
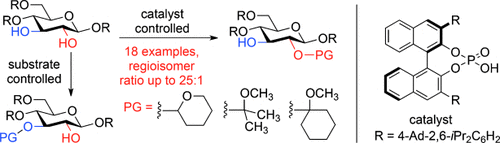
Graphical Abstract
In control: A chiral phosphoric acid catalyst (see scheme) significantly enhances or completely overrides the inherent regioselective acetalization profiles exhibited by monosaccharide-derived 1,2-diol substrates. This study represents the first example of chiral-catalyst-directed regio- and enantioselective intermolecular acetalizations, which are complementary to existing methods for substrate-controlled functionalization of polyols.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304298_sm_miscellaneous_information.pdf5.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. David, S. Hanessian, Tetrahedron 1985, 41, 643;
- 1bS. V. Ley, D. K. Baeschlin, D. J. Dixon, A. C. Foster, S. J. Ince, H. W. M. Priepke, D. J. Reynolds, Chem. Rev. 2001, 101, 53;
- 1cD. Lee, M. S. Taylor, Synthesis 2012, 3421.
- 2
- 2aC. A. Lewis, S. J. Miller, Angew. Chem. 2006, 118, 5744; Angew. Chem. Int. Ed. 2006, 45, 5616;
- 2bC. A. Lewis, J. Merkel, S. J. Miller, Bioorg. Med. Chem. Lett. 2008, 18, 6007;
- 2cT. Kawabata, W. Muramatsu, T. Nishio, T. Shibata, H. Schedel, J. Am. Chem. Soc. 2007, 129, 12890;
- 2dW. Muramatsu, K. Mishiro, Y. Ueda, T. Furuta, T. Kawabata, Eur. J. Org. Chem. 2010, 827;
- 2eP. A. Jordan, K. J. Kayser-Bricker, S. J. Miller, Proc. Natl. Acad. Sci. USA 2010, 107, 20620;
- 2fC. M. Longo, Y. Wei, M. F. Roberts, S. J. Miller, Angew. Chem. 2009, 121, 4222; Angew. Chem. Int. Ed. 2009, 48, 4158; Angew. Chem. 2009, 121, 4222;
- 2gK. W. Fiori, A. L. A. Puchlopek, S. J. Miller, Nat. Chem. 2009, 1, 630;
- 2hB. S. Fowler, K. M. Laemmerhold, S. J. Miller, J. Am. Chem. Soc. 2012, 134, 9755;
- 2iY. Zhao, J. Rodrigo, A. H. Hoveyda, M. L. Snapper, Nature 2006, 443, 67;
- 2jY. Zhao, A. W. Mitra, A. H. Hoveyda, M. L. Snapper, Angew. Chem. 2007, 119, 8623; Angew. Chem. Int. Ed. 2007, 46, 8471;
- 2kA. D. Worthy, X. Sun, K. L. Tan, J. Am. Chem. Soc. 2012, 134, 7321;
- 2lA.-M. R. Dechert-Schmitt, D. C. Schmitt, M. J. Krische, Angew. Chem. 2013, 125, 3277;
10.1002/ange.201209863 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 3195.
- 3
- 3aM. Rueping, B. J. Nachtsheim, W. Ieawsuwan, I. Atodiresei, Angew. Chem. 2011, 123, 6838;
10.1002/ange.201100169 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 6706;
- 3bR. J. Phipps, G. L. Hamilton, F. D. Toste, Nat. Chem. 2012, 4, 603;
- 3cK. Brak, E. N. Jacobsen, Angew. Chem. 2013, 125, 558;
10.1002/ange.201205449 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 534;
- 3dM. Mahlau, B. List, Angew. Chem. 2013, 125, 540;
10.1002/ange.201205343 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 518.
- 4
- 4aM. Terada, H. Tanaka, K. Sorimachi, J. Am. Chem. Soc. 2009, 131, 3430;
- 4bI. Čorić, S. Vellalath, B. List, J. Am. Chem. Soc. 2010, 132, 8536;
- 4cI. Čorić, S. Muller, B. List, J. Am. Chem. Soc. 2010, 132, 17370;
- 4dZ.-Y. Han, R. Guo, P.-S. Wang, D.-F. Chen, H. Xiao, L.-Z. Gong, Tetrahedron Lett. 2011, 52, 5963;
- 4eI. Čorić, B. List, Nature 2012, 483, 315;
- 4fZ. Sun, G. A. Winschel, A. Borovika, P. Nagorny, J. Am. Chem. Soc. 2012, 134, 8074–8077;
- 4gM. Terada, Y. Toda, Angew. Chem. 2012, 124, 2135; Angew. Chem. Int. Ed. 2012, 51, 2093;
- 4hH. Wu, Y.-P. He, L.-Z. Gong, Org. Lett. 2013, 15, 460;
- 4iP. Nagorny, Z. Sun, G. A. Winschel, Synlett 2013, 661;
- 4jJ. H. Kim, I. Coric, S. Vellalath, B. List, Angew. Chem. 2013, 125, 4570; Angew. Chem. Int. Ed. 2013, 52, 4474;
- 4kA. Borovika, P. Nagorny, Tetrahedron 2013, 69, 5719.
- 5P. J. Edwards, D. A. Entwistle, C. Genicot, S. V. Ley, G. Visentin, Tetrahedron: Asymmetry 1994, 5, 2609.
- 6Y. Demizu, Y. Kubo, H. Miyoshi, T. Maki, Y. Matumura, N. Moriyama, O. Onomura, Org. Lett. 2008, 10, 5075–5077.
- 7
- 7aL. Chan, M. S. Taylor, Org. Lett. 2011, 13, 3090;
- 7bD. Lee, M. S. Taylor, J. Am. Chem. Soc. 2011, 133, 3724;
- 7cC. Gouliaras, D. Lee, L. Chan, M. S. Taylor, J. Am. Chem. Soc. 2011, 133, 13926;
- 7dD. Lee, C. L. Williamson, L. Chan, M. S. Taylor, J. Am. Chem. Soc. 2012, 134, 8260.
- 8
- 8aK. Geyer, J. D. C. Codee, P. H. Seeberger, Chem. Eur. J. 2006, 12, 8434;
- 8bP. H. Seeberger, Chem. Soc. Rev. 2008, 37, 19;
- 8cR. R. Schmidt, Angew. Chem. 2009, 121, 1932; Angew. Chem. Int. Ed. 2009, 48, 1900.
- 9
- 9aD. J. Cox, M. D. Smith, A. J. Fairbanks, Org. Lett. 2010, 12, 1452;
- 9bE. I. Balmond, D. M. Coe, M. C. Galan, E. M. McGarrigle, Angew. Chem. 2012, 124, 9286;
10.1002/ange.201204505 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 9152.
- 10
- 10aG. J. F. Chittenden, Carbohydr. Res. 1971, 16, 495;
- 10bJ. Kihlberg, T. Frejd, K. Jansson, G. Magnusson, Carbohydr. Res. 1986, 152, 113.
- 11
- 11aJ. Kihlberg, T. Frejd, K. Jansson, S. Kitzing, G. Magnusson, Carbohydr. Res. 1989, 185, 171.
- 12
- 12aY. Tsuda, M. Nishimura, T. Kobayashi, Y. Sato, K. Kanemitsu, Chem. Pharm. Bull. 1991, 39, 2883;
- 12bC. M. Nycholat, D. R. Bundle, Carbohydr. Res. 2009, 344, 555.
- 13
- 13aR. Schwalm, H. Binder, D. Funhoff, J. Appl. Polym. Sci. 2000, 78, 208;
- 13bH. Kusama, R. Hara, S. Kawahara, T. Nishimori, H. Kashima, N. Nakamura, K. Morihira, I. Kowajima, J. Am. Chem. Soc. 2000, 122, 3811.
- 14aP. G. M. Wuts, T. W. Greene, Green’s Protective Groups in Organic Synthesis, 4th ed., Wiley, Hoboken, 2007, pp. 59–79;
- 14bP. J. Kocienski, Protecting Groups, 3rd ed., Georg Thieme, Stuttgart, 2005, pp. 315–320.
- 15
- 15aV. Amarnath, A. D. Broom, Chem. Rev. 1977, 77, 183;
- 15bC. B. Reese, Tetrahedron 1978, 34, 3143;
- 15cS. L. Beaucage, R. P. Iyer, Tetrahedron 1992, 48, 2223.
- 16M. Kotke, P. R. Schreiner, Synthesis 2007, 779.
- 17
- 17aM. Fleischmann, D. Drettwan, E. Sugiono, M. Rueping, R. M. Gschwind, Angew. Chem. 2011, 123, 6488;
10.1002/ange.201101385 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 6364.
- 18E. Kattnig, M. Albert, Org. Lett. 2004, 6, 945.
- 19H. Fujioka, Y. Nagatomi, H. Kitagawa, Y. Kita, J. Am. Chem. Soc. 1997, 119, 12016.