Zinc-Catalyzed Synthesis of Functionalized Furans and Triarylmethanes from Enynones and Alcohols or Azoles: Dual XH Bond Activation by Zinc†
Jesús González
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)
Search for more papers by this authorDr. Javier González
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)
Search for more papers by this authorCarmela Pérez-Calleja
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)
Search for more papers by this authorCorresponding Author
Dr. Luis A. López
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)Search for more papers by this authorCorresponding Author
Dr. Rubén Vicente
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)Search for more papers by this authorJesús González
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)
Search for more papers by this authorDr. Javier González
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)
Search for more papers by this authorCarmela Pérez-Calleja
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)
Search for more papers by this authorCorresponding Author
Dr. Luis A. López
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)Search for more papers by this authorCorresponding Author
Dr. Rubén Vicente
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)
Departamento de Química Orgánica e Inorgánica e Instituto Universitario de Química Organometálica “Enrique Moles”, Universidad de Oviedo c/Julián Clavería 8, 33007 Oviedo (Spain)Search for more papers by this authorFinancial support from the Ministerio de Economía y Competitividad of Spain (Grant CTQ2012-20517-C02-01 and predoctoral grant for J.G.) is gratefully acknowledged. Calculations were carried out at the “Cluster de Modelización Científica” (Universidad de Oviedo). R.V. is a Ramón y Cajal Fellow.
Graphical Abstract
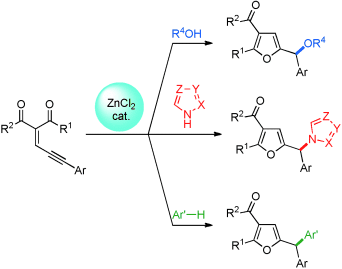
Ba'zinc'ga! A zinc-catalyzed sequence involving a cyclization with a subsequent CO, CN, or CC bond formation enables the preparation of a variety of valuable furfuryl ethers (with alcohols) and unsymmetrically substituted triarylmethane derivatives (with azoles or arenes). ZnCl2 serves as the catalyst.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201301625_sm_miscellaneous_information.pdf6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Applications of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective (Eds.: ), Wiley, Hoboken, 2012;
- 1b Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals, (Eds.: ), Wiley-VCH, Weinheim, 2004.
- 2
- 2a Catalysis without Precious Metals (Ed.: ), Wiley-VCH, Weinheim, 2010;
- 2bC. Bolm, J. Legros, J. Le Paih, L. Zani, Chem. Rev. 2004, 104, 6217;
- 2cS. Enthaler, K. Junge, M. Beller, Angew. Chem. 2008, 120, 3363;
10.1002/ange.200800012 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3317.
- 3Selected recent reviews on zinc catalysis:
- 3aS. Enthaler, ACS Catal. 2013, 3, 150;
- 3bX.-F. Wu, Chem. Asian J. 2012, 7, 2502;
- 3cX.-F. Wu, H. Neumann, Adv. Synth. Catal. 2012, 354, 3141.
- 4R. Vicente, J. González, L. Riesgo, J. González, L. A. López, Angew. Chem. 2012, 124, 8187;
10.1002/ange.201203914 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 8063.
- 5For reviews on furans, see:
- 5aH. N. C. Wong, X.-L. Hou, K.-S. Yeung, H. Huang in Modern Heterocyclic Chemistry (Eds.: ), Wiley-VCH, Weinheim, 2011;
10.1002/9783527637737.ch6 Google Scholar
- 5bA. Boto, L. Álvarez in Heterocycles in Natural Products Syntheis (Eds.: ), Wiley-VCH, Weinheim, 2011;
10.1002/9783527634880.ch4 Google Scholar
- 5cR. C. D. Brown, Angew. Chem. 2005, 117, 872; Angew. Chem. Int. Ed. 2005, 44, 850;
- 5dB. A. Keay, P. W. Dibble in Comprehensive Heterocyclic Chemistry II, Vol. 2 (Eds.: ), Elsevier, Oxford, 1997, p. 395.
- 6Selected examples:
- 6aX. Cui, X. Xu, L. Wojtas, M. M. Kim, X. P. Zhang, J. Am. Chem. Soc. 2012, 134, 19981;
- 6bC. H. Woo, P. M. Beaujuge, T. W. Holcombe, O. P. Lee, J. M. J. Fréchet, J. Am. Chem. Soc. 2010, 132, 15547;
- 6cO. Gidron, Y. Diskin-Posner, M. Bendikov, J. Am. Chem. Soc. 2010, 132, 2148;
- 6dA. Sniady, A. Durham, M. Morreale, A. Marcinek, S. Szafert, T. Lis, K. R. Brzezniska, T. Iwasaki, T. Ohshima, K. Mashima, R. Dembinski, J. Org. Chem. 2008, 73, 5881.
- 7For selected examples on the alkyne activation with zinc compounds, see:
- 7aA. Zulys, M. Dochnahl, D. Hollmann, K. Löhnwitz, J.-S. Herrmann, P. W. Roesky, S. Blechert, Angew. Chem. 2005, 117, 7972;
10.1002/ange.200502006 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7794;
- 7bA. Sniady, A. Durham, M. S. Morreale, K. A. Wheeler, R. Dembinsky, Org. Lett. 2007, 9, 1175;
- 7cK. Alex, A. Tillack, N. Schwarz, M. Beller, Angew. Chem. 2008, 120, 2337;
10.1002/ange.200703823 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2304;
- 7dC.-L. Deng, R.-J. Song, Y.-L. Liu, J.-H. Li, Adv. Synth. Catal. 2009, 351, 3096;
- 7eT. Sugiishi, H. Nakamura, J. Am. Chem. Soc. 2012, 134, 2504.
- 8H. Lebel, J.-F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977.
- 9For conceptually different approaches to furfuryl alcohol derivatives, see:
- 9aJ. S. Clark, A. Boyer, A. Aimon, P. E. García, D. M. Lindsay, A. D. F. Symington, Y. Danoy, Angew. Chem. 2012, 124, 12294; Angew. Chem. Int. Ed. 2012, 51, 12128;
- 9bL. Albrecht, L. K. Ransborg, B. Gschwend, K. A. Jørgensen, J. Am. Chem. Soc. 2010, 132, 17886.
- 10When using butanol and cyclopentanemethanol, variable amounts (ca. 5 %) of isomeric acetals were isolated. See the Supporting Information for details.
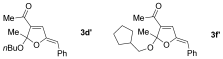
- 11Selected reviews on TRAMs:
- 11aM. S. Shchepinov, V. A. Korshun, Chem. Soc. Rev. 2003, 32, 170;
- 11bD. F. Duxbury, Chem. Rev. 1993, 93, 381. Selected examples of relevant TRAMs:
- 11cJ. Doiron, A.-H. Soultan, R. Richard, M. M. Touré, N. Picot, R. Richard, M. Cuperlovic-Culf, G. A. Robichaud, M. Touaibia, Eur. J. Med. Chem. 2011, 46, 4010;
- 11dR. Palchaudhuri, V. Nesterenko, P. J. Hergenrother, J. Am. Chem. Soc. 2008, 130, 10274;
- 11eA. V. Cheltsov, M. Aoyagi, A. Aleshin, E. C.-W. Yu, T. Gilliland, D. Zhai, A. A. Bobkov, J. C. Reed, R. C. Liddington, R. Abagyan, J. Med. Chem. 2010, 53, 3899;
- 11fM. R. Detty, S. L. Gibson, S. J. Wagner, J. Med. Chem. 2004, 47, 3897;
- 11gM. Irie, J. Am. Chem. Soc. 1983, 105, 2078.
- 12Methodologies to prepare unsymmetrical TRAMs are scarce. See selected examples:
- 12aJ. Zhang, A. Bellomo, A. D. Creamer, S. D. Dreher, P. J. Walsh, J. Am. Chem. Soc. 2012, 134, 13765;
- 12bB. L. H. Taylor, M. R. Harris, E. R. Jarvo, Angew. Chem. 2012, 124, 7910; Angew. Chem. Int. Ed. 2012, 51, 7790;
- 12cF.-Q. Yuan, L.-X. Gao, F.-S. Han, Chem. Commun. 2011, 47, 5289;
- 12dY.-Z. Li, B.-J. Li, X.-Y. Lu, S. Lin, Z.-J. Shi, Angew. Chem. 2009, 121, 3875; Angew. Chem. Int. Ed. 2009, 48, 3817;
- 12eB.-F. Shi, N. Maugel, Y.-H. Zhang, J.-Q. Yu, Angew. Chem. 2008, 120, 4960; Angew. Chem. Int. Ed. 2008, 47, 4882;
- 12fJ. Esquivias, R. G. Arrayás, J. C. Carretero, Angew. Chem. 2006, 118, 645;
10.1002/ange.200503305 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 629;
- 12gI. Iovel, K. Mertins, J. Kischel, A. Zapf, M. Beller, Angew. Chem. 2005, 117, 3981;
10.1002/ange.200462522 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3913.
- 13
- 13aG. Parkin, Chem. Rev. 2004, 104, 699;
- 13bK. A. McCall, C.-C. Huang, C. A. Fierke, J. Nutr. 2000, 130, 1437S;
- 13cH. Vahrenkamp, Acc. Chem. Res. 1999, 32, 589;
- 13dT. Koike, S. Kajitani, I. Nakamura, E. Kimura, M. Shiro, J. Am. Chem. Soc. 1995, 117, 1210. For a recently proposed similar role of zinc, see:
- 13eY. Wang, L. Liu, L. Zhang, Chem. Sci. 2013, 4, 739.
- 14For an acid-catalyzed furan formation from enynones, see: C. P. Casey, N. A. Strotman, J. Org. Chem. 2005, 70, 2576.
- 15When Brønsted acids were used for the reaction of 1 a with MeOH, the sole formation of 4 a was observed (see the Supporting Information). These results indicate the need for zinc to accomplish the formation of 3. For a previous report on the dimerization of enynones, see: J. Barluenga, L. Riesgo, R. Vicente, L. A. López, M. Tomás, J. Am. Chem. Soc. 2007, 129, 7772.
- 16The calculations were carried out with the Gaussian 09, Revision B.01, suite of programs, M. J. Frisch et al. Gaussian 09, revision B.01; Gaussian, Inc., Wallingford, CT, 2009. See the Supporting Information for details.
- 17See the Supporting Information for further details on the mechanism.
- 18For a review, see:
- 18aG. A. Olah, R. Krishnamurti, G. K. Surya Prakash in Comprehensive Organic Synthesis, Vol. 3 (Eds.: ), Pergamon, Oxford, 1991, p. 293. For recent related reports on Friedel–Crafts alkylations with zinc, see:
10.1016/B978-0-08-052349-1.00065-2 Google Scholar
- 18bA. G. Smith, J. S. Johnson, Org. Lett. 2010, 12, 1784;
- 18cC.-R. Liu, M.-B. Li, C.-F. Yang, S.-K. Tian, Chem. Eur. J. 2009, 15, 793;
- 18dW. Rao, P. W. H. Chan, Org. Biomol. Chem. 2008, 6, 2426.
- 19This transformation did not occur in the absence of MeOH.