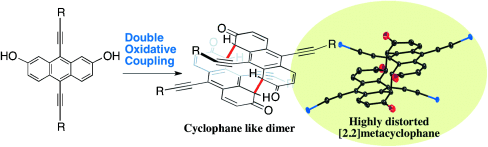
Synthesis of Highly Distorted π-Extended [2.2]Metacyclophanes by Intermolecular Double Oxidative Coupling†
Yutaro Koyama
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Aichi, 464-8603 (Japan)
Search for more papers by this authorCorresponding Author
Dr. Satoru Hiroto
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Aichi, 464-8603 (Japan)
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Aichi, 464-8603 (Japan)Search for more papers by this authorCorresponding Author
Prof. Dr. Hiroshi Shinokubo
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Aichi, 464-8603 (Japan)
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Aichi, 464-8603 (Japan)Search for more papers by this authorYutaro Koyama
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Aichi, 464-8603 (Japan)
Search for more papers by this authorCorresponding Author
Dr. Satoru Hiroto
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Aichi, 464-8603 (Japan)
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Aichi, 464-8603 (Japan)Search for more papers by this authorCorresponding Author
Prof. Dr. Hiroshi Shinokubo
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Aichi, 464-8603 (Japan)
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Aichi, 464-8603 (Japan)Search for more papers by this authorThis work was supported by Grants-in-Aid for Scientific Research (Nos. 22750036 and 24350023) from MEXT (Japan). H.S. also acknowledges the Toyoaki Scholarship Foundation for financial support.
Graphical Abstract
A strained relationship: Oxidation of dihydroxy-substituted acenes provides face-to-face [2.2]metacyclophane-like dimers (see scheme; O red, Si of iPr3Si groups blue). The products exhibited highly distorted structures caused by steric repulsion. UV/Vis and electrochemical analysis revealed that the HOMO–LUMO gap was decreased upon dimerization.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201301180_sm_miscellaneous_information.pdf4.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Fragments of Fullerenes and Carbon Nanotubes: Designed Synthesis Unusual Reactions, and Coordination Chemistry (Eds.: ), Wiley, Hoboken, 2012;
- 1b Fullerenes: Principles and Applications (Eds.: ), RSC, Cambridge, 2007;
- 1c Carbon-Rich Compounds: From Molecules to Materials (Eds.: ), Wiley-VCH, Weinhim, 2006.
- 2
- 2aH. Omachi, Y. Segawa, K. Itami, Acc. Chem. Res. 2012, 45, 1378;
- 2bA. Sygula, Eur. J. Org. Chem. 2011, 1611;
- 2cS. Higashibayashi, H. Sakurai, Chem. Lett. 2011, 40, 122;
- 2dT. Amaya, T. Hirao, Chem. Commun. 2011, 47, 10524;
- 2eV. M. Tsefrikas, L. T. Scott, Chem. Rev. 2006, 106, 4868;
- 2fY.-T. Wu, J. S. Siegel, Chem. Rev. 2006, 106, 4843.
- 3For recent breakthroughs for the synthesis of distorted π-conjugated compounds, see:
- 3aT.-C. Wu, M.-K. Chen, Y.-W. Lee, M.-Y. Kuo, Y.-T. Wu, Angew. Chem. 2013, 125, 1327; Angew. Chem. Int. Ed. 2013, 52, 1289;
- 3bL. T. Scott, E. A. Jackson, Q. Zhang, B. D. Steinberg, M. Bancu, B. Li, J. Am. Chem. Soc. 2012, 134, 107;
- 3cY. Segawa, S. Miyamoto, H. Omachi, S. Matsuura, P. Senel, T. Sasamori, N. Tokitoh, K. Itami, Angew. Chem. 2011, 123, 3302; Angew. Chem. Int. Ed. 2011, 50, 3244;
- 3dR. Jasti, J. Bhattachrjee, J. B. Neaton, C. R. Bertozzi, J. Am. Chem. Soc. 2008, 130, 17646;
- 3eS. Yamago, Y. Watanabe, T. Iwamoto, Angew. Chem. 2010, 122, 769;
10.1002/ange.200905659 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 757;
- 3fB. L. Merner, L. N. Dawe, G. J. Bodwell, Angew. Chem. 2009, 121, 5595;
10.1002/ange.200806363 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 5487.
- 4
- 4a Modern Cyclophane Chemistry (Eds.: ), Wiley-VCH, Weinheim, 2004;
- 4bF. Vötle, Cyclophane Chemistry, Wiley-VCH, New York, 1993;
- 4cH. Hopf, Angew. Chem. 2008, 120, 9954;
10.1002/ange.200800969 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9808.
- 5
- 5aG. P. Bartholomew, I. Ledoux, S. Mukamel, G. C. Bazan, J. Zyss, J. Am. Chem. Soc. 2002, 124, 13480;
- 5bW. Nakanishi, S. Hitosugi, A. Piskareva, Y. Shimada, H. Taka, H. Kita, H. Isobe, Angew. Chem. 2010, 122, 7397; Angew. Chem. Int. Ed. 2010, 49, 7239;
- 5cS. P. Jagtap, D. M. Collard, J. Am. Chem. Soc. 2010, 132, 12208;
- 5dY. Morisaki, Y. Chujo, Bull. Chem. Soc. Jpn. 2009, 82, 1070.
- 6D. Ramaiah, P. P. Neelakandan, A. K. Nair, R. R. Avirah, Chem. Soc. Rev. 2010, 39, 4158.
- 7L. Valentini, F. Mengoni, J. M. Kenny, A. Marrocchi, A. Taticchi, Small 2007, 3, 1200.
- 8
- 8aW. Baker, J. F. W. McOmie, J. M. Norman, J. Chem. Soc. 1951, 1114;
- 8bW. D. Rohrbach, F. Gerson, R. Möckel, V. Boekelheide, J. Org. Chem. 1984, 49, 4128.
- 9
- 9aK. Ayub, R. Li, C. Bohne, R. V. Williams, R. H. Mitchell, J. Am. Chem. Soc. 2011, 133, 4040;
- 9bR. V. Williams, W. D. Edwards, R. H. Mitchell, S. G. Robinson, J. Am. Chem. Soc. 2005, 127, 16207;
- 9cP. A. Liddell, G. Kodis, J. Andréasson, L. Garza, S. Bandyopadhyay, R. H. Mitchell, T. A. Moore, A. L. Moore, D. Gust, J. Am. Chem. Soc. 2004, 126, 4803;
- 9dY. Ting, Y.-H. Lai, J. Am. Chem. Soc. 2004, 126, 909;
- 9eG. J. Bodwell, J. N. Bridson, T. J. Houghton, J. W. J. Kennedy, M. R. Mannion, Angew. Chem. 1996, 108, 1418;
10.1002/ange.19961081211 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1320.
- 10
- 10aW. Baker, F. Glockling, J. F. W. McOmie, J. Chem. Soc. 1951, 1118;
- 10bW. Jenny, R. Peter, Angew. Chem. 1965, 77, 1027;
10.1002/ange.19650772255 Google ScholarAngew. Chem. Int. Ed. Engl. 1965, 4, 979;
- 10cF. Vötle, H. A. Staab, Chem. Ber. 1968, 101, 2709;
- 10dM. Sauer, H. A. Staab, Liebigs Ann. Chem. 1984, 615.
- 11H. Musso, Angew. Chem. 1963, 75, 965; Angew. Chem. Int. Ed. Engl. 1963, 2, 723, and references therein.
- 12For recent examples for the oxidation of phenol, see:
- 12aM. Uyanik, T. Mutsuga, K, Ishihara, Molecules 2012, 17, 8604;
- 12bA. Kirste, M. Nieger, I. M. Malkowsky, F. Stecker, A. Fischer, S. R. Waldvogel, Chem. Eur. J. 2009, 15, 2273.
- 13
- 13aH. Egami, K. Matsumoto, T. Oguma, T. Kunisu, T. Katsuki, J. Am. Chem. Soc. 2010, 132, 13633;
- 13bH. Wang, Chirality 2010, 22, 827;
- 13cJ. M. Brunel, Chem. Rev. 2005, 105, 857;
- 13dT. Chen, S. Yekta, A. K. Yudin, Chem. Rev. 2003, 103, 3155.
- 14A few examples of oxidation of hydroxy-substituted anthracenes have been reported; see: H. Laatsch, Liebigs Ann. Chem. 1986, 839.
- 15S. Hiroto, I. Hisaki, H. Shinokubo, A. Osuka, J. Am. Chem. Soc. 2008, 130, 16172.
- 16Crystallographic data for 2 a: C74H98O4Cl6Si4, Mr=1376.58, tetragonal, space group P43212, a=b=19.4656(10), c=19.711(2) Å, V=7468.8(9) Å3, Z=4, ρcalcd=1.224 g cm−3, R=0.0416 (I>2.0σ(I)), Rw=0.1117 (all data), GOF=1.095.[27]
- 17Crystallographic data for 2 b: C53H48O4Cl3, Mr=855.26, monoclinic, space group C2/c, a=16.127(5), b=14.313(5), c=20.389(7) Å, β=109.146(7)°, V=4466(3) Å3, Z=4, ρcalcd=1.278 g cm−3, R=0.0876 (I>2.0σ(I)), Rw=0.2509 (all data), GOF=1.065.[27]
- 18Crystallographic data for 2 c: C60H64O4, Mr=849.11, triclinic, space group P
 , a=8.936(4), b=10.057(4), c=14.039(5) Å, α=71.113(6), β=78.297(7), γ=84.127(6)°, V=1168.1(8) Å3, Z=1, ρcalcd=1.207 g cm−3, R=0.0495 (I>2.0σ(I)), Rw=0.1477 (all data), GOF=1.041.[27]
, a=8.936(4), b=10.057(4), c=14.039(5) Å, α=71.113(6), β=78.297(7), γ=84.127(6)°, V=1168.1(8) Å3, Z=1, ρcalcd=1.207 g cm−3, R=0.0495 (I>2.0σ(I)), Rw=0.1477 (all data), GOF=1.041.[27]
- 19F. Vögtle, P. Neumann, Angew. Chem. 1972, 84, 75;
10.1002/ange.19720840302 Google ScholarAngew. Chem. Int. Ed. Engl. 1972, 11, 73.
- 20Geometry optimization with a wB97XD functional resulted in better agreement with the X-ray crystal structures than other DFT methods, such as B3LYP;
- 20aS. M. Bachrach, J. Phys. Chem. A 2011, 115, 2396;
- 20bG. F. Caramori, S. E. Galembeck, J. Phys. Chem. A 2008, 112, 11784.
- 21H. J. Reich, D. J. Cram, J. Am. Chem. Soc. 1969, 91, 3517.
- 22
- 22aT. Kimoto, K. Tanaka, Y. Sakai, A. Ohno, K. Yoza, K. Kobayashi, Org. Lett. 2009, 11, 3658;
- 22bR. Ozawa, K. Yoza, K. Kobayashi, Chem. Lett. 2011, 40, 941.
- 23Crystallographic data for 5: C90H106O4Cl6Si4, Mr=1576.81, monoclinic, space group P21/c, a=12.285(2), b=13.464(3), c=26.580(5) Å, β=101.257(4)°, V=4312.0(14) Å3, Z=2, ρcalcd=1.214 g cm−3, R=0.0448 (I>2.0σ(I)), Rw=0.1418 (all data), GOF=1.024.[27]
- 24
- 24aS. Mukhopadhyay, S. P. Jagtap, V. Coropceanu, J.-L. Brédas, D. M. Collard, Angew. Chem. 2012, 124, 11797;
10.1002/ange.201205738 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11629;
- 24bS. P. Jagtap, S. Mukhopadhyay, V. Coropceanu, G. L. Brizius, J.-L. Brédas, D. M. Collard, J. Am. Chem. Soc. 2012, 134, 7176.
- 25The calculations with wB97XD/6-31G(d) afforded overestimated energies of the HOMO–LUMO gap for all of the molecules herein.
- 26In contrast to 5, the HOMO and LUMO of 1 a indicated no through-space overlap between two benzene moieties, suggesting the presence of through-space non-orbital interactions, such as columbic repulsion (Supporting Information, Figure S43).
- 27CCDC 922823 (2 a), CCDC 922824 (2 b), 922825 (2 c), and 922826 (5) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.