The Molecular Mechanism of Enzymatic Glycosyl Transfer with Retention of Configuration: Evidence for a Short-Lived Oxocarbenium-Like Species†
We thank Prof. A. Planas, Prof. B. G. Davis, and Dr. S. S. Lee for enlightening discussions on the mechanisms of GTs. This work was funded by the MICINN (FIS2008-03845) and GENCAT (2009SGR-1309). A.A. thanks MEC for a FPU studentship. We acknowledge the computer support, technical expertise, and assistance provided by the Barcelona Supercomputing Center-Centro Nacional de Supercomputación (BSC-CNS).
Graphical Abstract
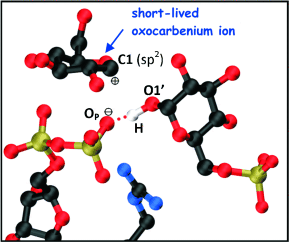
A quantum leap: By means of quantum mechanics/molecular mechanics metadynamics simulations, a front-face SNi-type reaction for glycosyl transfer with retention of the anomeric configuration is shown to be feasible. A short-lived oxocarbenium-like species (see picture; O red, P gold, N blue, C black) is identified and provides the complete itinerary of this long sought after molecular mechanism.