Achieving Secondary Structural Resolution in Kinetic Measurements of Protein Folding: A Case Study of the Folding Mechanism of Trp-cage†
Robert M. Culik
Department of Biochemistry and Molecular Biophysics, University of Pennsylvania (USA)
These authors contributed equally.
Search for more papers by this authorArnaldo L. Serrano
Department of Chemistry, University of Pennsylvania, 231 S. 34 Street, Philadelphia, PA 19104 (USA)
These authors contributed equally.
Search for more papers by this authorCorresponding Author
Prof. Dr. Michelle R. Bunagan
Department of Chemistry, College of New Jersey, 2000 Pennington Road, Ewing, NJ 08628 (USA)
Michelle R. Bunagan, Department of Chemistry, College of New Jersey, 2000 Pennington Road, Ewing, NJ 08628 (USA)
Feng Gai, Department of Chemistry, University of Pennsylvania, 231 S. 34 Street, Philadelphia, PA 19104 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng Gai
Department of Chemistry, University of Pennsylvania, 231 S. 34 Street, Philadelphia, PA 19104 (USA)
Michelle R. Bunagan, Department of Chemistry, College of New Jersey, 2000 Pennington Road, Ewing, NJ 08628 (USA)
Feng Gai, Department of Chemistry, University of Pennsylvania, 231 S. 34 Street, Philadelphia, PA 19104 (USA)
Search for more papers by this authorRobert M. Culik
Department of Biochemistry and Molecular Biophysics, University of Pennsylvania (USA)
These authors contributed equally.
Search for more papers by this authorArnaldo L. Serrano
Department of Chemistry, University of Pennsylvania, 231 S. 34 Street, Philadelphia, PA 19104 (USA)
These authors contributed equally.
Search for more papers by this authorCorresponding Author
Prof. Dr. Michelle R. Bunagan
Department of Chemistry, College of New Jersey, 2000 Pennington Road, Ewing, NJ 08628 (USA)
Michelle R. Bunagan, Department of Chemistry, College of New Jersey, 2000 Pennington Road, Ewing, NJ 08628 (USA)
Feng Gai, Department of Chemistry, University of Pennsylvania, 231 S. 34 Street, Philadelphia, PA 19104 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng Gai
Department of Chemistry, University of Pennsylvania, 231 S. 34 Street, Philadelphia, PA 19104 (USA)
Michelle R. Bunagan, Department of Chemistry, College of New Jersey, 2000 Pennington Road, Ewing, NJ 08628 (USA)
Feng Gai, Department of Chemistry, University of Pennsylvania, 231 S. 34 Street, Philadelphia, PA 19104 (USA)
Search for more papers by this authorWe thank the National Institutes of Health (GM-065978, RR01348, and GM-008275) for funding. R.M.C. acknowledges a training grant in structural biology.
Graphical Abstract
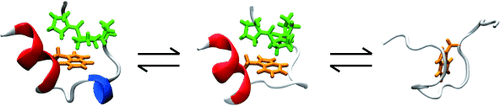
A new twist: A multi-probe and multi-frequency approach is shown for dissecting the folding dynamics of individual protein structural elements. In response to a temperature jump the 310-helix (blue in the picture) of the miniprotein Trp-cage unfolds before the global unfolding of the protein, whereas the formation of the cage structure depends on the folding of the α-helix (red).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201104085_sm_miscellaneous_information.pdf144.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. W. Neidigh, R. M. Fesinmeyer, N. H. Andersen, Nat. Struct. Biol. 2002, 9, 425–430.
- 2C. D. Snow, B. Zagrovic, V. S. Pande, J. Am. Chem. Soc. 2002, 124, 14548–14549.
- 3C. Simmerling, B. Strockbine, A. E. Roitberg, J. Am. Chem. Soc. 2002, 124, 11258–11259.
- 4S. Chowdhury, M. C. Lee, G. M. Xiong, Y. Duan, J. Mol. Biol. 2003, 327, 711–717.
- 5J. W. Pitera, W. Swope, Proc. Natl. Acad. Sci. USA 2003, 100, 7587–7592.
- 6G. V. Nikiforovich, N. H. Andersen, R. M. Fesinmeyer, C. Frieden, Proteins Struct. Funct. Genet. 2003, 52, 292–302.
- 7A. Schug, T. Herges, W. Wenzel, Phys. Rev. Lett. 2003, 91, 158102.
- 8P. Carnevali, G. Toth, G. Toubassi, S. N. Meshkat, J. Am. Chem. Soc. 2003, 125, 14244–14245.
- 9S. Chowdhury, M. C. Lee, Y. Duan, J. Phys. Chem. B 2004, 108, 13855–13865.
- 10P. J. Steinbach, Proteins Struct. Funct. Genet. 2004, 57, 665–677.
- 11M. Ota, M. Ikeguchi, A. Kidera, Proc. Natl. Acad. Sci. USA 2004, 101, 17658–17663.
- 12A. Linhananta, J. Boer, I. MacKay, J. Chem. Phys. 2005, 122, 114901–114915.
- 13A. S. N. Seshasayee, Theor. Biol. Med. Modell. 2005, 2, 7–11.
- 14F. Ding, S. V. Buldyrev, N. V. Dokholyan, Biophys. J. 2005, 88, 147–155.
- 15A. Irbäck, S. Mohanty, Biophys. J. 2005, 88, 1560–1569.
- 16J. L. Alonso, P. Echenique, Biophys. Chem. 2005, 115, 159–168.
- 17J. Chen, W. Im, C. L. Brooks, J. Am. Chem. Soc. 2006, 128, 3728–3736.
- 18L. X. Zhan, J. Z. Y. Chen, W. K. Liu, Proteins Struct. Funct. Genet. 2007, 66, 436–443.
- 19D. Paschek, H. Nymeyer, A. E. Garcia, J. Struct. Biol. 2007, 157, 524–533.
- 20L. Yang, M. P. Grubb, Y. Q. Gao, J. Chem. Phys. 2007, 126, 125102.
- 21D. A. C. Beck, G. W. N. White, V. Daggett, J. Struct. Biol. 2007, 157, 514–523.
- 22S. Piana, A. Laio, J. Phys. Chem. B 2007, 111, 4553–4559.
- 23J. Copps, R. F. Murphy, S. Lovas, Biopolymers 2007, 88, 427–437.
- 24A. Kentsis, T. Gindin, M. Mezei, R. Osman, PLoS ONE 2007, 2, e 446.
- 25Z. H. Hu, Y. H. Tang, H. F. Wang, X. Zhang, M. Lei, Arch. Biochem. Biophys. 2008, 475, 140–147.
- 26P. Hudáky, P. Straner, V. Farkas, G. Varadi, G. Toth, A. Perczel, Biochemistry 2008, 47, 1007–1016.
- 27W. X. Xu, Y. G. Mu, Biophys. Chem. 2008, 137, 116–125.
- 28D. Paschek, S. Hempel, A. E. Garcia, Proc. Natl. Acad. Sci. USA 2008, 105, 17754–17759.
- 29S. Wu, P. I. Zhuravlev, G. A. Papoian, Biophys. J. 2008, 95, 5524–5532.
- 30X. Q. Yao, Z. S. She, Biochem. Biophys. Res. Commun. 2008, 373, 64–68.
- 31S. Kannan, M. Zacharias, Proteins Struct. Funct. Genet. 2009, 76, 448–460.
- 32J. Cerný, J. Vondrasek, P. Hobza, J. Phys. Chem. B 2009, 113, 5657–5660.
- 33Y. Chebaro, X. Dong, R. Laghaei, P. Derreumaux, N. Mousseau, J. Phys. Chem. B 2009, 113, 267–274.
- 34D. Matthes, B. L. de Groot, Biophys. J. 2009, 97, 599–608.
- 35F. Marinelli, F. Pietrucci, A. Laio, S. Piana, PLoS Comput. Biol. 2009, 5, e 1000452.
- 36Z. Gattin, S. Riniker, P. J. Hore, K. H. Mok, W. F. van Gunsteren, Protein Sci. 2009, 18, 2090–2099.
- 37M. Gao, H. Q. Zhu, X. Q. Yao, Z. S. She, Biochem. Biophys. Res. Commun. 2010, 392, 95–99.
- 38C. Velez-Vega, E. E. Borrero, F. A. Escobedo, J. Chem. Phys. 2010, 133, 105103.
- 39N. J. Bruce, R. A. Bryce, J. Chem. Theory Comput. 2010, 6, 1925–1930.
- 40M. S. Lee, M. A. Olson, J. Chem. Theory Comput. 2010, 6, 2477–2487.
- 41R. Day, D. Paschek, A. E. Garcia, Proteins Struct. Funct. Genet. 2010, 78, 1889–1899.
- 42W. Zheng, E. Gallicchio, N. Deng, M. Andrec, R. M. Levy, J. Phys. Chem. B 2011, 115, 1512–1523.
- 43L. L. Qiu, S. A. Pabit, A. E. Roitberg, S. J. Hagen, J. Am. Chem. Soc. 2002, 124, 12952–12953.
- 44M. R. Bunagan, X. Yang, J. G. Saven, F. Gai, J. Phys. Chem. B 2006, 110, 3759–3763.
- 45H. Neuweiler, S. Doose, M. Sauer, Proc. Natl. Acad. Sci. USA 2005, 102, 16650–16655.
- 46K. H. Mok, L. T. Kuhn, M. Goez, I. J. Day, J. C. Lin, N. H. Andersen, P. J. Hore, Nature 2007, 447, 106–109.
- 47Z. Ahmed, I. A. Beta, A. V. Mikhonin, S. A. Asher, J. Am. Chem. Soc. 2005, 127, 10943–10950.
- 48R. H. Zhou, Proc. Natl. Acad. Sci. USA 2003, 100, 13280–13285.
- 49C. Y. Huang, Z. Getahun, Y. J. Zhu, J. W. Klemke, W. F. Degrado, F. Gai, Proc. Natl. Acad. Sci. USA 2002, 99, 2788–2793.
- 50L. Tadesse, R. Nazarbaghi, L. Walters, J. Am. Chem. Soc. 1991, 113, 7036–7037.
- 51S. M. Decatur, J. Antonic, J. Am. Chem. Soc. 1999, 121, 11914–11915.
- 52C. Y. Huang, Z. Getahun, T. Wang, W. F. Degrado, F. Gai, J. Am. Chem. Soc. 2001, 123, 12111–12112.
- 53B. Barua, J. C. Lin, V. D. Williams, P. Kummler, J. W. Neidigh, N. H. Andersen, Protein Eng. Des. Sel. 2008, 21, 171–185.
- 54A. R. Fersht, A. Matouschek, L. Serrano, J. Mol. Biol. 1992, 224, 771–782.
- 55A. Barth, C. Zscherp, Q. Rev. Biophys. 2002, 35, 369–430.
- 56D. V. Williams, A. Byrne, J. Stewart, N. H. Andersen, Biochemistry 2011, 50, 1143–1152.
- 57S. Bagchi, C. Falvo, S. Mukamel, R. M. Hochstrasser, J. Phys. Chem. B 2009, 113, 11260–11273.
- 58D. F. Kennedy, M. Crisma, C. Toniolo, D. Chapman, Biochemistry 1991, 30, 6541–6548.
- 59R. A. G. D. Silva, S. C. Yasui, J. Kubelka, F. Formaggio, M. Crisma, C. Toniolo, T. A. Keiderling, Biopolymers 2002, 65, 229–243.
- 60S. Williams, T. P. Causgrove, R. Gilmanshin, K. S. Fang, R. H. Callender, W. H. Woodruff, R. B. Dyer, Biochemistry 1996, 35, 619–697.
- 61P. A. Thompson, W. A. Eaton, J. Hofrichter, Biochemistry 1997, 36, 9200–9210.
- 62C. Y. Huang, J. W. Klemke, Z. Getahun, W. F. DeGrado, F. Gai, J. Am. Chem. Soc. 2001, 123, 9235–9238.
- 63R. H. Zhou, J. Mol. Graphics Modell. 2004, 22, 451–463.
- 64J. Juraszek, P. G. Bolhuis, Proc. Natl. Acad. Sci. USA 2006, 103, 15859–15864.
- 65Y. Xu, R. Oyola, F. Gai, J. Am. Chem. Soc. 2003, 125, 15388–15394.
- 66A. L. Serrano, M. J. Tucker, F. Gai, J. Phys. Chem. B 2011, 115, 7472–7478.
- 67S. Mukherjee, P. Chowdhury, M. R. Bunagan, F. Gai, J. Phys. Chem. B 2008, 112, 9146–9150.
- 68H. Maity, M. Maity, M. M. G. Krishna, L. Mayne, S. W. Englander, Proc. Natl. Acad. Sci. USA 2005, 102, 4741–4746.