Solid-State Conversion of the Solvated Dimer [{tBuZn(μ-OtBu)(thf)}2] into a Long Overlooked Trimeric [{tBuZnOtBu}3] Species†
Prof. Dr. Janusz Lewiński
Department of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw (Poland), Fax: (+48) 22-234-7279 http://lewin.ch.pw.edu.pl
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw (Poland)
Search for more papers by this authorMichał Dutkiewicz
Department of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw (Poland), Fax: (+48) 22-234-7279 http://lewin.ch.pw.edu.pl
Search for more papers by this authorMichał Lesiuk
Department of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw (Poland), Fax: (+48) 22-234-7279 http://lewin.ch.pw.edu.pl
Search for more papers by this authorDr. Witold Śliwiński
Department of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw (Poland), Fax: (+48) 22-234-7279 http://lewin.ch.pw.edu.pl
Search for more papers by this authorDr. Karolina Zelga
Department of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw (Poland), Fax: (+48) 22-234-7279 http://lewin.ch.pw.edu.pl
Search for more papers by this authorDr. Iwona Justyniak
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw (Poland)
Search for more papers by this authorProf. Dr. Janusz Lipkowski
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw (Poland)
Search for more papers by this authorProf. Dr. Janusz Lewiński
Department of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw (Poland), Fax: (+48) 22-234-7279 http://lewin.ch.pw.edu.pl
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw (Poland)
Search for more papers by this authorMichał Dutkiewicz
Department of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw (Poland), Fax: (+48) 22-234-7279 http://lewin.ch.pw.edu.pl
Search for more papers by this authorMichał Lesiuk
Department of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw (Poland), Fax: (+48) 22-234-7279 http://lewin.ch.pw.edu.pl
Search for more papers by this authorDr. Witold Śliwiński
Department of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw (Poland), Fax: (+48) 22-234-7279 http://lewin.ch.pw.edu.pl
Search for more papers by this authorDr. Karolina Zelga
Department of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw (Poland), Fax: (+48) 22-234-7279 http://lewin.ch.pw.edu.pl
Search for more papers by this authorDr. Iwona Justyniak
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw (Poland)
Search for more papers by this authorProf. Dr. Janusz Lipkowski
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw (Poland)
Search for more papers by this authorWe gratefully acknowledge funding from the Ministry of Science and Higher Education (Grant No. N204 164336), the European Union (within the European Regional Development Fund through the grant Innovative Economy POIG.01.01.02-00-008/08) (I.J.,M.D., and M.L.), and the Foundation for Polish Science (K.Z.). We are grateful to Prof. Z. Kaszkur for PXRD measurements.
Graphical Abstract
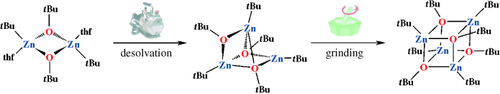
Zinc links: Solid-state desolvation of the dimeric alkoxide [{tBuZn(μ-OtBu)(thf)}2] leads to a trimer [{tBuZnOtBu}3] with a unique core structure; subsequent grinding affords the tetrameric cubane [{tBuZnOtBu}4]. This approach demonstrates a new direction in the generation of metal alkoxide clusters.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201004504_sm_miscellaneous_information.pdf741.3 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. Frankland, Justus Liebigs Ann. 1849, 71, 171–213;
10.1002/jlac.18490710205 Google Scholar
- 1bE. Frankland, J. Chem. Soc. 1850, 2, 263–296.
10.1039/QJ8500200263 Google Scholar
- 2
- 2aM. M. Olmstead, P. P. Power, S. C. Shoner, J. Am. Chem. Soc. 1991, 113, 3379–3385;
- 2bJ. Boersma, Comprehensive Organometallic Chemistry, Vol. 2 (Eds.: ), Pergamon, Oxford, 1984, chap. 16.
- 3
- 3aK. Su, T. D. Tilley, M. J. Sailor, J. Am. Chem. Soc. 1996, 118, 3459–3468;
- 3bC. G. Kim, K. Sung, T. M. Chung, D. Y. Jung, Y. Kim, Chem. Commun. 2003, 2068–2069;
- 3cS. Polarz, A. Roy, M. Merz, S. Halm, D. Schröder, L. Schneider, G. Bacher, F. E. Kruis, M. Driess, Small 2005, 1, 540–552;
- 3dT. J. Boyle, S. D. Bunge, N. L. Andrews, L. E. Matzen, K. Sieg, M. A. Rodriguez, T. J. Headley, Chem. Mater. 2004, 16, 3279–3288;
- 3eS. Polarz, J. Strunk, V. Ischenko, M. W. E. van den Berg, O. Hinrichsen, M. Muhler, M. Driess, Angew. Chem. 2006, 118, 3031–3035;
10.1002/ange.200503068 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2965–2969;
- 3fC. Lizandara-Pueyo, M. W. E. van den Berg, A. De Toni, T. Goes, S. Polarz, J. Am. Chem. Soc. 2008, 130, 16601–16610;
- 3gT. Hikov, A. Rittermeier, M. B. Luedemann, C. Herrmann, M. Muhler, R. A. Fischer, J. Mater. Chem. 2008, 18, 3325–3331.
- 4R. Steudel, Y. Steudel, J. Phys. Chem. A 2006, 110, 8912–8924.
- 5In the solid state, dimeric structures have only been observed for sterically encumbered aryloxy groups bridging between two zinc atoms:
- 5aM. H. Chisholm, J. C. Gallucci, H. Yin, H. Zhen, Inorg. Chem. 2005, 44, 4777–4785;
- 5bS. Enthaler, B. Eckhardt, S. Inoue, E. Irran, M. Driess, Chem. Asian J. 2010, 5, 2027–2035.
- 6
- 6aH. M. M. Shearer, C. B. Spencer, Chem. Commun. 1966, 194–194;
- 6bY. Matsui, K. Kamiya, M. Nishikawa, Bull. Chem. Soc. Jpn. 1966, 39, 1828–1828;
- 6cH. M. M. Shearer, C. B. Spencer, Acta Crystallogr. Sect. B 1980, 36, 2046–2050;
- 6dA. B. Charette, C. Molinaro, C. Brochu, J. Am. Chem. Soc. 2001, 123, 12160–12167;
- 6eS. Jana, R. J. F. Berger, R. Fröhlich, T. Pape, N. W. Mitzel, Inorg. Chem. 2007, 46, 4293–4297.
- 7
- 7aM. Driess, K. Merz, S. Rell, Eur. J. Inorg. Chem. 2000, 2517–2522;
- 7bG. Anantharaman, H. W. Roesky, J. Magull, Angew. Chem. 2002, 114, 1274–1277;
10.1002/1521-3757(20020402)114:7<1274::AID-ANGE1274>3.0.CO;2-5 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1226–1229;10.1002/1521-3773(20020402)41:7<1226::AID-ANIE1226>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 7cK. Merz, H. M. Hu, S. Rell, M. Driess, Eur. J. Inorg. Chem. 2003, 51–53.
- 8
- 8aJ. Lewiński, W. Marciniak, I. Justyniak, J. Lipkowski, J. Am. Chem. Soc. 2003, 125, 12698–12699;
- 8bJ. Lewiński, K. Suwała, M. Kubisiak, Z. Ochal, I. Justyniak, J. Lipkowski, Angew. Chem. 2008, 120, 8006–8009; Angew. Chem. Int. Ed. 2008, 47, 7888–7891.
- 9K. Ruhlandt-Senge, P. P. Power, Inorg. Chem. 1993, 32, 4505–4508.
- 10G. E. Coates, P. D. Roberts, J. Chem. Soc. A 1967, 1233–1234.
- 11J. G. Noltes, J. Boersma, J. Organomet. Chem. 1968, 12, 425–431.
- 12We also note that an equilibrium between tetrameric and dimeric species was observed for [{MeZnOtBu}n]: J. M. Bruce, B. C. Cutsforth, D. W. Farren, F. G. Hutchinson, F. M. Rabagliati, D. R. Reed, J. Chem. Soc. B 1966, 1020–1024.
- 13J. Lewiński, M. Dranka, W. Bury, W. Śliwiński, I. Justyniak, J. Lipkowski, J. Am. Chem. Soc. 2007, 129, 3096–3098.
- 14J. Lewiński, W. Śliwiński, M. Dranka, I. Justyniak, J. Lipkowski, Angew. Chem. 2006, 118, 4944–4947; Angew. Chem. Int. Ed. 2006, 45, 4826–4829.
- 15
- 15aD. Braga, F. Grepioni, Angew. Chem. 2004, 116, 4092–4102;
10.1002/ange.200301721 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4002–4011;
- 15bA. L. Garay, A. Pichon, S. L. James, Chem. Soc. Rev. 2007, 36, 846–855;
- 15cG. Kaupp, CrystEngComm 2009, 11, 388–403;
- 15dD. Braga, F. Grepioni, L. Maini, Chem. Commun. 2010, 46, 6232–6242;
- 15eT. Friščić, J. Mater. Chem. 2010, 20, 7599–7605.
- 16For an example concerning thermolysis of the molecular [{R2Ga(L)}2](i-OC6H4O) adduct in the solid state resulting in the loss of the Lewis base L and the formation of gallium aryloxide polymer, see: L. H. van Poppel, S. G. Bott, A. R. Barron, J. Am. Chem. Soc. 2003, 125, 11006–11017; for related studies involving transition-metal complexes, see:
- 16aJ. Yoshida, S. Nishikiori, R. Kuroda, Chem. Eur. J. 2008, 14, 10570–10578;
- 16bR. Kuroda, J. Yoshida, A. Nakamura, S. Nishikiori, CrystEngComm 2009, 11, 427–432;
- 16cA. S. R. Chesman, D. R. Turner, G. B. Deacon, S. R. Batten, Chem. Commun. 2010, 46, 4899–4901.
- 17For selected examples utilizing mechanochemical procedures in the preparation of metal clusters, see:
- 17aV. Chandrasekhar, V. Baskar, R. Boomishankar, K. Gopal, S. Zacchini, J. F. Bickley, A. Steiner, Organometallics 2003, 22, 3710–3716;
- 17bA. Orita, L. Jiang, T. Nakano, N. Ma, J. Otera, Chem. Commun. 2002, 1362–1363;
- 17cF. Strobridge, N. Juda, T. Friščić, CrystEngComm 2010, 12, 2409–2418;
- 17dS. Perruchas, X. F. Le Goff, S. Maron, I. Maurin, F. Guillen, A. Garcia, T. Gacoin, J.-P. Boilot, J. Am. Chem. Soc. 2010, 132, 10967–10969.
- 18For details of optimized geometries, energies, and more information about calculations, see the Supporting Information.
- 19G. M. Sheldrick, Acta Crystallogr. Sect. A 1990, 46, 467–473.
- 20G. M. Sheldrick, SHELXL-97, Program for Structure Refinement, Universität Göttingen, 1997.