A {Cu2S}2+ Mixed-Valent Core Featuring a CuCu Bond†
Dr. Stéphane Torelli
Laboratoire de Chimie et Biologie des Métaux, Université Joseph Fourier, CNRS, UMR 5249, CEA, DSV/iRTSV/LCBM Bat K', 17, avenue des Martyrs, 38054 Grenoble Cedex 9 (France), Fax: (+33) 4-3878-9124
Search for more papers by this authorDr. Maylis Orio
Equipe de Chimie Théorique, Département de Chimie Moléculaire, Université Joseph Fourier, CNRS, UMR 5250, B.P. 53, 38041 Grenoble Cedex 9 (France)
Search for more papers by this authorDr. Jacques Pécaut
Laboratoire de Reconnaissance Ionique et Chimie de Coordination—(UMR-E 3 CEA-UJF), SCIB/INAC, CEA-Grenoble, 17, avenue des Martyrs, 38054 Grenoble Cedex 9 (France)
Search for more papers by this authorDr. Hélène Jamet
Equipe de Chimie Théorique, Département de Chimie Moléculaire, Université Joseph Fourier, CNRS, UMR 5250, B.P. 53, 38041 Grenoble Cedex 9 (France)
Search for more papers by this authorDr. Laurent Le Pape
Laboratoire de Chimie et Biologie des Métaux, Université Joseph Fourier, CNRS, UMR 5249, CEA, DSV/iRTSV/LCBM Bat K', 17, avenue des Martyrs, 38054 Grenoble Cedex 9 (France), Fax: (+33) 4-3878-9124
Search for more papers by this authorDr. Stéphane Ménage
Laboratoire de Chimie et Biologie des Métaux, Université Joseph Fourier, CNRS, UMR 5249, CEA, DSV/iRTSV/LCBM Bat K', 17, avenue des Martyrs, 38054 Grenoble Cedex 9 (France), Fax: (+33) 4-3878-9124
Search for more papers by this authorDr. Stéphane Torelli
Laboratoire de Chimie et Biologie des Métaux, Université Joseph Fourier, CNRS, UMR 5249, CEA, DSV/iRTSV/LCBM Bat K', 17, avenue des Martyrs, 38054 Grenoble Cedex 9 (France), Fax: (+33) 4-3878-9124
Search for more papers by this authorDr. Maylis Orio
Equipe de Chimie Théorique, Département de Chimie Moléculaire, Université Joseph Fourier, CNRS, UMR 5250, B.P. 53, 38041 Grenoble Cedex 9 (France)
Search for more papers by this authorDr. Jacques Pécaut
Laboratoire de Reconnaissance Ionique et Chimie de Coordination—(UMR-E 3 CEA-UJF), SCIB/INAC, CEA-Grenoble, 17, avenue des Martyrs, 38054 Grenoble Cedex 9 (France)
Search for more papers by this authorDr. Hélène Jamet
Equipe de Chimie Théorique, Département de Chimie Moléculaire, Université Joseph Fourier, CNRS, UMR 5250, B.P. 53, 38041 Grenoble Cedex 9 (France)
Search for more papers by this authorDr. Laurent Le Pape
Laboratoire de Chimie et Biologie des Métaux, Université Joseph Fourier, CNRS, UMR 5249, CEA, DSV/iRTSV/LCBM Bat K', 17, avenue des Martyrs, 38054 Grenoble Cedex 9 (France), Fax: (+33) 4-3878-9124
Search for more papers by this authorDr. Stéphane Ménage
Laboratoire de Chimie et Biologie des Métaux, Université Joseph Fourier, CNRS, UMR 5249, CEA, DSV/iRTSV/LCBM Bat K', 17, avenue des Martyrs, 38054 Grenoble Cedex 9 (France), Fax: (+33) 4-3878-9124
Search for more papers by this authorWe thank Colette Lebrun for assistance in recording mass spectra and Lionel Dubois for access to the UV/NIR spectrophotometer.
Graphical Abstract
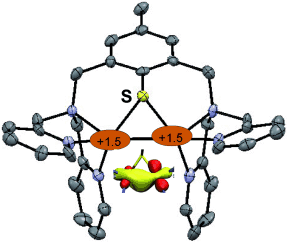
Not so far away from me: Reductive cleavage of a disulfide ligand with CuI leads to the formation of a new mixed-valent CuIICuI complex (see picture). Combined X-ray and theoretical investigations highlight the presence of a striking Cu2S core containing a CuCu bond, and solution studies also indicate a high degree of delocalization.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201003411_sm_miscellaneous_information.pdf457.4 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. A. Lewis, W. B. Tolman, Chem. Rev. 2004, 104, 1047–1076.
- 2R. Balasubramanian, S. M. Smith, S. Rawat, L. A. Yatsunyk, T. M. Stemmler, A. C. Rosenzweig, Nature 2010, 465, 115–119.
- 3S. Iwata, C. Ostermeier, B. Ludwig, H. Michel, Nature 1995, 376, 660–669.
- 4T. Tsukihara, H. Aoyama, E. Yamashita, T. Tomikazi, H. Yamaguchi, K. Shinzawa-Itoh, R. Nakashima, R. Yaono, S. Yoshikawa, Science 1995, 269, 1069–1074.
- 5M. Wilmanns, P. Lappalainen, M. Kelly, E. Sauer-Eriksson, M. Saraste, Proc. Natl. Acad. Sci. USA 1995, 92, 11955–11959.
- 6E. Kim, E. E. Chufán, K. Kamaraj, K. D. Karlin, Chem. Rev. 2004, 104, 1077–1133.
- 7K. Brown, M. Tegon, M. Prudencio, A. S. Pereira, S. Besson, J. J. G. Moura, I. Moura, M. Tegoni, C. Cambillau, Nat. Struct. Biol. 2000, 7, 191–195.
- 8K. Brown, K. Djinovic-Carugo, T. Haltia, I. Cabrito, M. Saraste, J. J. G. Moura, I. Moura, M. Tegoni, C. Cambillau, J. Biol. Chem. 2000, 275, 41133–41136.
- 9K. Paraskevopoulos, S. V. Antonyuk, R. G. Sawers, R. R. Eady, S. S. Hasnain, J. Mol. Biol. 2006, 362, 55–65.
- 10T. Haltia, Brown, M. Tegoni, C. Cambillau, M. Saraste, K. Matilla, K. Djinovic-Carugo, Biochem. J. 2003, 369, 77–88.
- 11M. R. Robin, P. Day, Adv. Inorg. Chem. Radiochem. 1968, 10, 247–403.
- 12N. J. Blackburn, M. E. Barr, W. H. Woodruff, J. van der Oost, S. de Vries, Biochemistry 1994, 33, 10401–10407.
- 13N. J. Blackburn, S. de Vries, M. E. Barr, R. P. Houser, W. B. Tolman, D. Sanders, J. A. Fee, J. Am. Chem. Soc. 1997, 119, 6135–6143.
- 14R. P. Houser, V. G. Young, W. B. Tolman, J. Am. Chem. Soc. 1996, 118, 2101–2102.
- 15R. R. Gagné, C. A. Koval, T. J. Smith, J. Am. Chem. Soc. 1977, 99, 8367–8368.
- 16D. D. Lecloux, R. Davydov, S. J. Lippard, J. Am. Chem. Soc. 1998, 120, 6810–6811.
- 17D. D. Lecloux, R. Davydov, S. J. Lippard, Inorg. Chem. 1998, 37, 6814–6826, and references therein.
- 18R. Gupta, Z. H. Zhang, D. Powell, M. P. Hendrich, A. S. Borovik, Inorg. Chem. 2002, 41, 5100–5106.
- 19J. R. Hagadorn, T. I. Zahn, L. Que, Jr., W. B. Tolman, Dalton Trans. 2003, 1790–1794.
- 20J. Kuzelka, Mukhopadhyay, B. Spingler, S. J. Lippard, Inorg. Chem. 2004, 43, 1751–1761.
- 21S. Kababya, J. Nelson, C. Calle, F. Neese, D. Goldfarb, J. Am. Chem. Soc. 2006, 128, 2017–2029.
- 22P. Chen, I. Cabrito, J. J. G. Moura, I. Moura, E. I. Solomon, J. Am. Chem. Soc. 2002, 124, 10497–10507.
- 23S. Ghosh, S. I. Gorelsky, S. DeBeer George, J. M. Chan, I. Cabrito, D. M. Dooley, J. J. G. Moura, I. Moura, E. I. Solomon, J. Am. Chem. Soc. 2007, 129, 3955–3965.
- 24S. Itoh, M. Nagagawa, S. Fukuzumi, J. Am. Chem. Soc. 2001, 123, 4087–4088.
- 25Crystal data for 1-(CF3SO3)2: C35H33Cu2F6N6O6S3, Mr=970.93, crystal size 0.50 mm×0.04 mm×0.04 mm, monoclinic, space group P21/n, a=9.4703(7) Å, b=28.166(3) Å, c=14.3634(9) Å, β=91.82(5)°, V=3831(5) Å3, ρcalcd=1.683 g cm−3, Z=4, μ=1.357 mm−1, 2θmax=49.42, T=150(2) K, (λMoKα=0.71073 Å), F(0,0,0)=1972, 16 275 reflections collected, 6484 unique, Rint=0.0470. The final agreement factors are R1=0.065 and wR2=0.1917 for data with I>2σ(I). CCDC 777283 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 26Y. Fu, B.-L. Lin, K.-S. Song, L. Liu, Q.-X. Guo, J. Chem. Soc. Perkin Trans. 2 2002, 1223–1230.
- 27Onsager slope in CH3CN=1352 (Supporting Information, Figure S6), ESI MS (CH3CN): [M−CF3SO3]+=822.1 (Supporting Information, Figure S4).
- 28R. R. Gagné, C. A. Koval, T. J. Smith, M. C. Cimolino, J. Am. Chem. Soc. 1979, 101, 4571–4580.
- 291-(CF3SO3)2 in (CH3)2CO (λmax [nm] (ε [M−1 cm−1]): 355 (3150), 537 (1050), 816 (1650), 1017 (1190); in CH2Cl2: 365 (3250), 540 (755), 838 (1050), 1185 (720); in CH3CN : 382 (1600), 510 (465), 875 (675), 1310 (205).
- 30CuA (N2OR) from A. cycloclastes:[31] 350 (sh), 481 (5200), 534 (5300), 630 (sh), 780 (2900); CuA-soluble binding domain from P. denitrificans:[32] 363 (1200), 480 (3000), 532 (3000), 808 (1600); [(LiPrdacoSCu)2]2+ in CH3OH: 358(2700), 602(800), 786(sh), 1466 (1200); CuZ (N2OR) from P. nautica:[22] 356 (3295), 500 (930), 700 (1455), 638 (3470), 1000 (1760).
- 31C. L. Hulse, B. A. Averill, Biochem. Biophys. Res. Commun. 1990, 166, 729.
- 32P. Lappalainen, R. Aasa, B. G. Malström, M. Saraste, J. Biol. Chem. 1993, 268, 26416.
- 33P. Chen, S. I. Gorelsky, S. Ghosh, E. I. Solomon, Angew. Chem. 2004, 116, 4224–4233; Angew. Chem. Int. Ed. 2004, 43, 4132–4140.
- 34J. M. Chan, J. A. Bollinger, C. L. Grewell, D. M. Dooley, J. Am. Chem. Soc. 2004, 126, 3030–3031.
- 35I. Bar-Nahum, A. K. Gupta, S. M. Huber, M. Z. Ertem, C. J. Cramer, W. B. Tolman, J. Am. Chem. Soc. 2009, 131, 2812–2814.
- 36W. B. Tolman, Angew. Chem. 2010, 122, 1034–1041;
10.1002/ange.200905364 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 1018–1024.