Direct Catalytic Asymmetric Three-Component Kabachnik–Fields Reaction†
Xu Cheng Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2999
Search for more papers by this authorRichard Goddard Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2999
Search for more papers by this authorGernot Buth Dr.
ISS, Forschungszentrum Karlsruhe, Postfach 3640, 76021 Karlsruhe (Germany)
Search for more papers by this authorBenjamin List Prof. Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2999
Search for more papers by this authorXu Cheng Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2999
Search for more papers by this authorRichard Goddard Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2999
Search for more papers by this authorGernot Buth Dr.
ISS, Forschungszentrum Karlsruhe, Postfach 3640, 76021 Karlsruhe (Germany)
Search for more papers by this authorBenjamin List Prof. Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2999
Search for more papers by this authorWe thank Alfred Deege and Heike Hinrichs for HPLC measurements. This work was funded in part by the DFG (priority program “Organocatalysis” SPP1179). Generous support by the Max Planck Society, Novartis (Young Investigator Award to B.L.), the Fonds der Chemischen Industrie (Silver Award to B.L.), and AstraZeneca (Research Award in Organic Chemistry to B.L.) is gratefully acknowledged.
Graphical Abstract
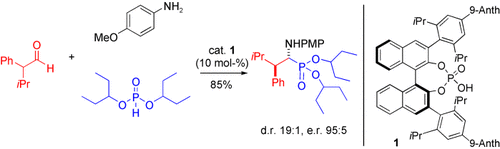
As mimics of α-amino acids, α-amino phosphonates have great promise as antibacterial and anti-HIV agents as well as protease inhibitors. Racemic α-branched aldehydes react, in the presence the new chiral phosphoric acid catalyst 1, directly with p-anisidine (PMPNH2) and a phosphite to furnish β-branched α-amino phosphonates highly diastereoselectively and enantioselectively. Anth=anthracenyl.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200801173_sm_miscellaneous_information.pdf9.5 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. B. Smith III, K. M. Yager, C. M. Taylor, J. Am. Chem. Soc. 1995, 117, 10879.
- 2
- 2aJ. G. Allen, F. R. Atherton, M. J. Hall, C. H. Hassal, S. W. Holmes, R. W. Lambert, L. J. Nisbet, P. S. Ringrose, Nature 1978, 272, 56;
- 2bF. R. Atherton, C. H. Hassall, R. W. Lambert, J. Med. Chem. 1986, 29, 29.
- 3E. Alonso, A. Solis, C. del Pozo, Synlett 2000, 698.
- 4R. Hirschmann, A. B. Smith III, C. M. Taylor, P. A. Benkovic, S. D. Taylor, K. M. Yager, P. A. Sprengler, S. J. Benkovics, Science 1994, 265, 234.
- 5For other biological applications of α-amino phosphonic acids, see:
- 5aM. C. Allen, W. Fuhrer, B. Tuch, R. Wade, J. M. Wood, J. Med. Chem. 1989, 32, 1652;
- 5bJ. Ding, M. E. Fraser, J. H. Meyer, P. A. Bartlett, M. N. G. James, J. Am. Chem. Soc. 1998, 120, 4610;
- 5cW. W. Smith, P. A. Bartlett, J. Am. Chem. Soc. 1998, 120, 4622;
- 5dJ. Bird, R. C. D. Mello, G. P. Harper, D. J. Hunter, E. H. Karran, R. E. Markwell, A. J. Miles-Williams, S. S. Rahman, R. W. Ward, J. Med. Chem. 1994, 37, 158.
- 6For reviews on non-catalytic variants, see:
- 6aB. Dhawan, D. Redmore, Phosphorus Sulfur Silicon Relat. Elem. 1987, 32, 119;
- 6bV. P. Kukhar, V. A. Soloshonok, V. A. Solodenko, Phosphorus Sulfur Silicon Relat. Elem. 1994, 92, 239;
- 6cO. I. Kolodiazhnyi, Tetrahedron: Asymmetry 1998, 9, 1279. For recent examples of auxiliary-controlled asymmetric hydrophosphonylations, see:
- 6dD. Enders, L. Tedeschi, J. W. Bats, Angew. Chem. 2000, 112, 4774;
10.1002/1521-3757(20001215)112:24<4774::AID-ANGE4774>3.0.CO;2-T Google ScholarAngew. Chem. Int. Ed. 2000, 39, 4605;10.1002/1521-3773(20001215)39:24<4605::AID-ANIE4605>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 6eF. A. Davis, S. Lee, H. Yan, D. D. Titus, Org. Lett. 2001, 3, 1757.
- 7For reviews on enantioselective catalytic hydrophosphonylations, see:
- 7aH. Gröger, B. Hammer, Chem. Eur. J. 2000, 6, 943;
10.1002/(SICI)1521-3765(20000317)6:6<943::AID-CHEM943>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 7bF. Palacios, C. Alonso, J. M. De Los Santos, Chem. Rev. 2005, 105, 899.
- 8
- 8aH. Sasai, S. Arai, Y. Tahara, M. Shibasaki, J. Org. Chem. 1995, 60, 6656;
- 8bH. Gröger, Y. Saida, H. Sasai, K. Yamaguchi, J. Martens, M. Shibasaki, J. Am. Chem. Soc. 1998, 120, 3089;
- 8cS. Kobayashi, H. Kiyohara, Y. Nakamura, R. J. Matsubara, J. Am. Chem. Soc. 2004, 126, 6558.
- 9G. D. Joly, E. N. Jacobsen, J. Am. Chem. Soc. 2004, 126, 4102.
- 10T. Akiyama, H. Morita, J. Itoh, K. Fuchibe, Org. Lett. 2005, 7, 2583.
- 11
- 11aD. Pettersen, M. Marcolini, L. Bernardi, F. Fini, R. P. Herrera, V. Sgarzani, A. Ricci, J. Org. Chem. 2006, 71, 6269;
- 11bJ. Wang, L. D. Heikkinen, H. Li, L. Zu, W. Jiang, H. Xie, W. Wang, Adv. Synth. Catal. 2007, 349, 1052.
- 12For a one-pot in situ sequence, see: B. Saito, H. Egami, T. Katsuki, J. Am. Chem. Soc. 2007, 129, 1978.
- 13For some leading references on chiral phosphoric acid catalysis, see:
- 13aT. Akiyama, J. Itoh, K. Yokota, K. Fuchibe, Angew. Chem. 2004, 116, 1592;
10.1002/ange.200353240 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1566;
- 13bD. Uraguchi, M. Terada, J. Am. Chem. Soc. 2004, 126, 5356;
- 13cS. Hoffmann, A. M. Seayad, B. List, Angew. Chem. 2005, 117, 7590;
10.1002/ange.200503062 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7424;
- 13dG. B. Rowland, H. Zhang, E. B. Rowland, S. Chennamadhavuni, Y. Wang, J. C. Antilla, J. Am. Chem. Soc. 2005, 127, 15696;
- 13eR. I. Storer, D. E. Carrera, Y. Ni, D. W. C. MacMillan, J. Am. Chem. Soc. 2006, 128, 84;
- 13fM. Rueping, E. Sugiono, C. Azap, Angew. Chem. 2006, 118, 2679;
10.1002/ange.200504344 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2617;
- 13gJ. Itoh, K. Fuchibe, T. Akiyama, Angew. Chem. 2006, 118, 4914; Angew. Chem. Int. Ed. 2006, 45, 4796;
- 13hQ. Kang, Z.-A. Zhao, S.-L. You, J. Am. Chem. Soc. 2007, 129, 1484;
- 13iQ.-X. Guo, H. Liu, C. Guo, S.-W. Luo, Y. Gu, L.-Z. Gong, J. Am. Chem. Soc. 2007, 129, 3790;
- 13jY.-X. Jia, J. Zhong, S.-F. Zhu, C.-M. Zhang, Q.-L. Zhou, Angew. Chem. 2007, 119, 5661; Angew. Chem. Int. Ed. 2007, 46, 5565;
- 13kM. J. Wanner, R. N. S. van der Haas, K. R. de Cuba, J. H. van Maarseveen, H. Hiemstra, Angew. Chem. 2007, 119, 7629;
10.1002/ange.200701808 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7485;
- 13lQ.-S. Guo, D.-M. Du, J. Xu, Angew. Chem. 2008, 120, 771; Angew. Chem. Int. Ed. 2008, 47, 759;
- 13mS.-M. Xu, Z. Wang, X. Zhang, X.-M. Zhang, K.-L. Ding, Angew. Chem. 2008, 120, 2882; Angew. Chem. Int. Ed. 2008, 47, 2480; for a review, see:
- 13nT. Akiyama, Chem. Rev. 2007, 107, 5744.
- 14S. Hoffmann, M. Nicoletti, B. List, J. Am. Chem. Soc. 2006, 128, 13074.
- 15For other TRIP-catalyzed reactions, see:
- 15aT. Akiyama, Y. Tamura, J. Itoh, H. Morita, K. Fuchibe, Synlett 2006, 141;
- 15bT. Akiyama, European Patent EP1623971, 2006;
- 15cJ. Seayad, A. M. Seayad, B. List, J. Am. Chem. Soc. 2006, 128, 1086;
- 15dM. Terada, K. Sorimachi, J. Am. Chem. Soc. 2007, 129, 292–293; for asymmetric counteranion-directed catalytic (ACDC) reactions involving TRIP, see:
- 15eS. Mayer, B. List, Angew. Chem. 2006, 118, 4299; Angew. Chem. Int. Ed. 2006, 45, 4193;
- 15fN. J. A. Martin, B. List, J. Am. Chem. Soc. 2006, 128, 13368;
- 15gJ. Zhou, B. List, J. Am. Chem. Soc. 2007, 129, 7498;
- 15hS. Mukherjee, B. List, J. Am. Chem. Soc. 2007, 129, 11336;
- 15iG. L. Hamilton, E. J. Kang, M. Mba, F. D. Toste, Science 2007, 317, 496;
- 15jX.-W. Wang, B. List, Angew. Chem. 2008, 120, 1135; Angew. Chem. Int. Ed. 2008, 47, 1119; for a review on the application of TRIP in catalysis, see:
- 15kG. Adair, S. Mukherjee, B. List, Aldrichimica Acta 2008, in press.
- 16
- 16aX. Qian, M. D. Shenderovich, K. E. Kövér, P. Davis, R. Horváth, T. Zalewska, H. I. Yamamura, F. Porreca, V. J. Hruby, J. Am. Chem. Soc. 1996, 118, 7280;
- 16bF. Huang, W. M. Nau, Angew. Chem. 2003, 115, 2371; Angew. Chem. Int. Ed. 2003, 42, 2269;
- 16cV. J. Hruby, J. Med. Chem. 2003, 46, 4215;
- 16dB. D. Zlatopolskiy, A. de Meijere, Chem. Eur. J. 2004, 10, 4718;
- 16eT. Saito, N. Iwata, S. Tsubuki, Y. Takaki, J. Takano, S.-M. Huang, T. Suemoto, M. Higuchi, T. C. Saido, Nat. Med. 2005, 11, 434.
- 17Alternative nitrogen sources that have been investigated include BocNH2, CbzNH2, benzamide, benzylamine, dibenzylamine, morpholine, and diphenylamine, which all gave no reaction. Among the anilines tested, p-anisidine gave higher enantioselectivities than p-hydroxylaniline, p-ethoxyaniline, p-phenoxyaniline, and o-methoxyaniline. We have also investigated other phospites, including diethyl-, diisopropyl-, and di(o-nitrobenzyl)phosphite and found di(3-pentyl)phosphite gave the highest enantioselectivities.
- 18
- 18aM. Rueping, A. P. Antonchick, C. Brinkmann, Angew. Chem. 2007, 119, 7027;
10.1002/ange.200702439 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6903;
- 18bT. Akiyama, H. Morita, K. Fuchibe, J. Am. Chem. Soc. 2006, 128, 13070;
- 18cJ. Itoh, K. Fuchibe, T. Akiyama, Angew. Chem. 2006, 118, 4914; Angew. Chem. Int. Ed. 2006, 45, 4796;
- 18dM. Terada, K. Machioka, K. Sorimachi, Angew. Chem. 2006, 118, 2312; Angew. Chem. Int. Ed. 2006, 45, 2254.
- 19For synthetic details, see the Supporting Information.
- 20CCDC-679478 (λ=1.54178 Å) and CCDC-679479 (λ=1.77121 Å) (4 g) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.