Reaction of Molecular Oxygen with an NHC-Coordinated Pd0 Complex: Computational Insights and Experimental Implications†
Brian V. Popp
Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706, USA, Fax: (+1) 608-262-6143
Search for more papers by this authorJohanna E. Wendlandt
Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706, USA, Fax: (+1) 608-262-6143
Search for more papers by this authorClark R. Landis Prof.
Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706, USA, Fax: (+1) 608-262-6143
Search for more papers by this authorShannon S. Stahl Prof.
Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706, USA, Fax: (+1) 608-262-6143
Search for more papers by this authorBrian V. Popp
Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706, USA, Fax: (+1) 608-262-6143
Search for more papers by this authorJohanna E. Wendlandt
Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706, USA, Fax: (+1) 608-262-6143
Search for more papers by this authorClark R. Landis Prof.
Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706, USA, Fax: (+1) 608-262-6143
Search for more papers by this authorShannon S. Stahl Prof.
Department of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706, USA, Fax: (+1) 608-262-6143
Search for more papers by this authorWe thank M. Konnick for providing (IMes)2Pd0. Financial support and computational resources from the following agencies and the UW Parallel Computing Center are gratefully acknowledged: NSF (CHE-0094344 and CHE-0091916), NCSA (CHE-060010T), and gifts from Intel Corporation. NHC=N-heterocyclic carbene.
Graphical Abstract
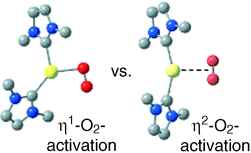
O2 activation: Computational studies of the reaction of O2 with an [(NHC)2Pd0] (NHC=N-heterocyclic carbene) complex reveal an unexpectedly small driving force for formation of a PdII(η2-O2) product. This result led to experimental demonstration of reversible O2 coordination to the (NHC)2Pd center. Computational analysis of the reaction coordinate reveals that O2 reacts with Pd0 through a stepwise mechanism involving an η1-O2 transition state.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z603667_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. S. Stahl, Science 2005, 309, 1824;
- 1bS. S. Stahl, Angew. Chem. 2004, 116, 3480;
10.1002/ange.200300630 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3400;
- 1cM. S. Sigman; M. J. Schultz, Org. Biomol. Chem. 2004, 2, 2551;
- 1dB. M. Stoltz, Chem. Lett. 2004, 33, 362;
- 1eT. Nishimura, S. Uemura, Synlett 2004, 201;
- 1fR. A. Sheldon, I. W. C. E. Arends, G.-J. ten Brink, A. Dijksman, Acc. Chem. Res. 2002, 35, 774;
- 1gM. Toyota, M. Ihara, Synlett 2002, 1211;
- 1hB. V. Popp, S. S. Stahl, Top. Organomet. Chem. 2007, 22, in press.
- 2For extensive discussion of this issue, see: B. A. Steinhoff, S. S. Stahl, J. Am. Chem. Soc. 2006, 128, 4348.
- 3
- 3aS. S. Stahl, J. L. Thorman, R. C. Nelson, M. A. Kozee, J. Am. Chem. Soc. 2001, 123, 7188;
- 3bM. M. Konnick, I. A. Guzei, S. S. Stahl, J. Am. Chem. Soc. 2004, 126, 10212.
- 4C. R. Landis, C. M. Morales, S. S. Stahl, J. Am. Chem. Soc. 2004, 126, 16302.
- 5
- 5aJ. M. Keith, R. J. Nielsen, J. Oxgaard, W. A. Goddard III, J. Am. Chem. Soc. 2005, 127, 13172;
- 5bT. Privalov, C. Linde, K. Zetterberg, C. Moberg, Organometallics 2005, 24, 885;
- 5cW. Zierkiewicz, T. Privalov, Organometallics 2005, 24, 6019;
- 5dR. J. Nielsen, W. A. Goddard III, J. Am. Chem. Soc. 2006, 128, 9651.
- 6M. Yamashita, K. Goto, T. Kawashima, J. Am. Chem. Soc. 2005, 127, 7294.
- 7The rates of individual steps in the catalytic cycle have, thus far, prevented direct observation of the Pd0 intermediate and its reaction with molecular oxygen.
- 8
- 8aM. P. Lanci, D. W. Brinkley, K. L. Stone, V. V. Smirnov, J. P. Roth, Angew. Chem. 2005, 117, 7439;
10.1002/ange.200502096 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7273;
- 8bV. V. Smirnov, D. W. Brinkley, M. P. Lanci, K. D. Karlin, J. P. Roth, J. Mol. Catal. A: Chem. 2006, 251, 100.
- 9
- 9aGaussian 03, Revision B.05 and D.01, M. J. Frisch, et al. Gaussian, Inc., Wallingford, CT, 2004. See the Supporting Information.
- 10T. Yoshida, S. Otsuka, J. Am. Chem. Soc. 1977, 99, 2134.
- 11
- 11aW. A. Herrmann, Angew. Chem. 2002, 114, 1342;
Angew. Chem. Int. Ed. 2002, 41, 1290;
10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 11bR. H. Crabtree, J. Organomet. Chem. 2005, 690, 5451;
- 11cR. Dorta, E. D. Stevens, N. M. Scott, C. Costabile, L. Cavallo, C. D. Hoff, S. P. Nolan, J. Am. Chem. Soc. 2005, 127, 2485.
- 12Initially, single-point solvation energy corrections were performed with the IEF-PCM model on the gas-phase geometries; however, analysis of the solvation-corrected MECP did not yield reasonable energies (see the Supporting Information). Although the energies of the other species seemed reasonable, we re-optimized all structures with the inclusion of the solvent model. This had a significant impact on the geometry of the MECP (see the Supporting Information).
- 13J. N. Harvey, M. Aschi, H. Schwarz, W. Koch, Theor. Chem. Acc. 1998, 99, 95.
- 14Location of a true transition state and MECP by gradient optimization was not possible owing to the constraints imposed during optimization. Optimizations performed without constraint result in formation of the lower-energy stationary points shown in Figure 1.
- 15The reaction of molecular oxygen with isoelectronic CuI complexes to form η2-peroxo adducts has been proposed to involve a related mechanism. See the following leading reference: N. W. Aboelella, S. V. Kryatov, B. F. Gherman, W. W. Brennessel, V. G. Young, Jr.,R. Sarangi, E. V. Rybak-Akimova, K. O. Hodgson, B. Hedman, E. I. Solomon, C. J. Cramer, W. B. Tolman, J. Am. Chem. Soc. 2004, 126, 16896–16911.
- 16J. P. Roth, J. P. Klinman in Isotope Effects in Chemistry and Biology (Eds.: ), CRC, Boca Raton, 2006, and references therein.
- 17Computational studies have played a critical role in the interpretation of other heavy-atom isotope effects. For a prominent example, see: A. J. DelMonte, J. Haller, K. N. Houk, K. B. Sharpless, D. A. Singleton, T. Strassner, A. A. Thomas, J. Am. Chem. Soc. 1997, 119, 9907.
- 18See the Supporting Information for details.
- 19Two imaginary frequencies were identified from the normal-mode analysis performed on the structure of TS′. The first mode (−77.9 cm−1) corresponds to a symmetric PdO2 “stretching” mode that resembles the side-on reaction coordinate in Figure 3. The second mode (−334.3 cm−1) exhibits atomic displacements in which one PdO bond shortens while the other lengthens, as if shifting the O2 fragment to an end-on binding mode, resembling the reaction coordinate in Figure 1.