Olefin-Metathesis Catalysts for the Preparation of Molecules and Materials (Nobel Lecture)†
Robert H. Grubbs Prof.
Victor and Elizabeth Atkins Professor of Chemistry Arnold and Mabel Beckman Laboratories of Chemical Synthesis, California Institute of Technology, Pasadena, CA 91125, USA, Fax: (+1) 626–564–9297
Search for more papers by this authorRobert H. Grubbs Prof.
Victor and Elizabeth Atkins Professor of Chemistry Arnold and Mabel Beckman Laboratories of Chemical Synthesis, California Institute of Technology, Pasadena, CA 91125, USA, Fax: (+1) 626–564–9297
Search for more papers by this authorCopyright The Nobel Foundation 2005. We thank the Nobel Foundation, Stockholm, for permission to print this lecture
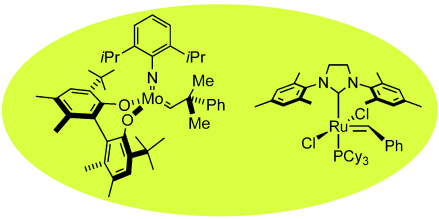
Graphical Abstract
Metathesis reactions are among the most important processes in organic synthesis. The decisive breakthrough in making these reactions practical for industrial purposes, which range from the synthesis of polymers to pharmaceuticals, came with the discovery of the reaction mechanism by Yves Chauvin and the targeted development of transition-metal-based metathesis catalysts by Richard Schrock and Robert Grubbs. The winners of the Chemistry Nobel Prize in 2005 present first-hand accounts of these developments.
References
- 1R. H. Grubbs, Tetrahedron 2004, 60, 7117; Handbook of Metathesis, Vol. 3 (Ed.: ), Wiley-VCH, Weinheim, 2003.
- 2 The Chain Straighteners: Fruitful Innovation: The Discovery of Linear and Steroregular Synthetic Polymers, McMillan, London, 1979.
- 3W. L. Truett, D. R. Johnson, I. M. Robinson, J. Am. Chem. Soc 1960, 2337.
- 4N. Calderon, E. A. Ofstead, J. P. Ward, W. A. Judy, K. W. Scott, J. Am. Chem. Soc. 1968, 90, 4133; R. L. Banks, G. C. Bailey, Ind. Eng. Chem. Prod. Res. Dev. 1964, 170.
- 5J. L. Hérisson, Y. Chauvin, Makromol. Chem. 1971, 141, 162; T. J. Katz, J. McGinnis, J. Am. Chem. Soc. 1975, 97, 1592; C. P. Casey, T. J. Burkhardt, J. Am. Chem. Soc. 1974, 96, 7808.
- 6R. H. Grubbs, D. D. Carr, C. Hoppin, P. L. Burk, J. Am. Chem. Soc. 1976, 98, 3478; R. H. Grubbs, P. L. Burk, D. D. Carr, J. Am. Chem. Soc. 1975, 97, 3265; T. J. Katz, R. Rothchild, J. Am. Chem. Soc. 1976, 98, 2519.
- 7T. R. Howard, J. B. Lee, R. H. Grubbs, J. Am. Chem. Soc. 1980, 102, 6876; S. H. Pine, R. Zahler, D. A. Evans, R. H. Grubbs, J. Am. Chem. Soc. 1980, 102, 3270; J. R. Stille, R. H. Grubbs, J. Am. Chem. Soc. 1986, 108, 855; F. N. Tebbe, G. W. Parshall, G. S. Reddy, J. Am. Chem. Soc. 1978, 100, 3611.
- 8L. R. Gilliom, R. H. Grubbs, J. Am. Chem. Soc. 1986, 108, 733; R. R. Schrock, J. Feldman, L. F. Cannizzo, R. H. Grubbs, Macromolecules 1987, 20, 1169.
- 9F. W. Michelotti, W. P. Keaveney, J. Polym. Sci. Part A 1965, 895.
- 10B. M. Novak, R. H. Grubbs, J. Am. Chem. Soc. 1988, 110, 960.
- 11S. T. Nguyen, L. K. Johnson, R. H. Grubbs, J. W. Ziller, J. Am. Chem. Soc. 1992, 114, 3974; S. T. Nguyen, R. H. Grubbs, J. W. Ziller, J. Am. Chem. Soc. 1993, 115, 9858.
- 12G. C. Fu, S. T. Nguyen, R. H. Grubbs, J. Am. Chem. Soc. 1993, 115, 9856; G. C. Fu, R. H. Grubbs, J. Am. Chem. Soc. 1992, 114, 7324.
- 13P. Schwab, M. B. France, J. W. Ziller, R. H. Grubbs, Angew. Chem. 1995, 107, 2179;
10.1002/ange.19951071818 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 2039; T. E. Welhelm, T. R. Belderrain, S. N. Brown, R. H. Grubbs, Organometallics 1997, 16, 3867.
- 14E. L. Dias, S. T. Nguyen, R. H. Grubbs, J. Am. Chem. Soc. 1997, 119, 3887; M. S. Sanford, M. Ulman, R. H. Grubbs, J. Am. Chem. Soc. 2001, 123, 749; M. S. Sanford, J. A. Love, R. H. Grubbs, J. Am. Chem. Soc. 2001, 123, 6543.
- 15M. Scholl, S. Ding. C. W. Lee, R. H. Grubbs, Org. Lett. 1999, 1, 953;
C. W. Bielawski, R. H. Grubbs, Angew. Chem. 2000, 112, 3025;
10.1002/1521-3757(20000818)112:16<3025::AID-ANGE3025>3.0.CO;2-F Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2903;10.1002/1521-3773(20000818)39:16<2903::AID-ANIE2903>3.0.CO;2-Q CAS PubMed Web of Science® Google ScholarT. M. Trnka, J. P Morgan, M. S. Sanford, T. E. Wilhelm, M. Scholl, T.-L. Choi, S. Ding, M. W. Day, R. H. Grubbs, J. Am. Chem. Soc. 2003, 125, 2546; J. A. Love, M. S. Sanford, M. W. Day, R. H. Grubbs, J. Am. Chem. Soc. 2003, 125, 10103.
- 16T. Nicola, M. Brenner, K. Donsbach, P. Kreye, Org. Process Res. Dev. 2005, 27; I. Kodota, H. Takamura, K. Sato, A. Ohno, K. Matusuda, Y. Yamamoto, J. Am. Chem. Soc. 2003, 125, 46.
- 17C. S. Woodson, Jr.,R. H. Grubbs, Patent No. 6,310,121 B1, October 30, 2001; C. S. Woodson, Jr.,R. H. Grubbs, U.S. Patent No. 5,939,504, August 17, 1999.
- 18J.-P. Sauvage, B. Mohr, R. H. Grubbs, M. Weck, Angew. Chem. 1997, 109, 1365;
10.1002/ange.19971091217 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1308; A. F. M. Kilbinger, S. J. Cantrill, A. W. Waltman, M. W. Day, R. H. Grubbs, Angew. Chem. 2003, 115, 3403; Angew. Chem. Int. Ed. 2003, 42, 3281; E. N. Guidry, S. J. Cantrill, J. F. Stoddart, R. H. Grubbs, Org. Lett. 2005, 7, 2129.
- 19Frost & Sullivan's Industrial Bioprocessing Alert, Sept. 9, 2005; http://www.epa.gov/greenchemistry/whats_gc.html.
- 20R. L. Pederson, R. H. Grubbs, U.S. Patent No. 6,696,597, February 24, 2004; R. L. Pederson, R. H. Grubbs, U.S. Patent No. 6,215,019 B1, April 3, 2001.