Sequential Enzymatic Oxidation of Aminoarenes to Nitroarenes via Hydroxylamines†
We thank the Deutsche Forschungsgemeinschaft for financial support in the priority program SPP1152 “Evolution of Metabolic Diversity” (HE 3469/2) and A. Perner for MS measurements.
Graphical Abstract
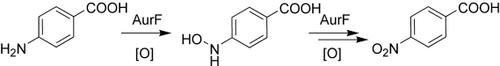
The at-line detection of an amino group oxidation catalyzed by the N-oxygenase AurF provides the first direct evidence for a stepwise enzymatic oxidation of aromatic amines to nitro groups via a hydroxylamine intermediate (see scheme). The sequential nature of this reaction is further supported by biotransformation of the intermediate and the identification of a p-aminobenzoate-derived azoxy side product.